Eicosanoids definition
Eicosanoids are biologically active lipid derivatives of unsaturated fatty acids containing 20 carbons. Eicosanoids are locally acting bioactive hormones that act near the point of hormone synthesis and included in the class of paracrine hormones. disease. Eicosanoids are derived from arachidonic acid and related polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA).
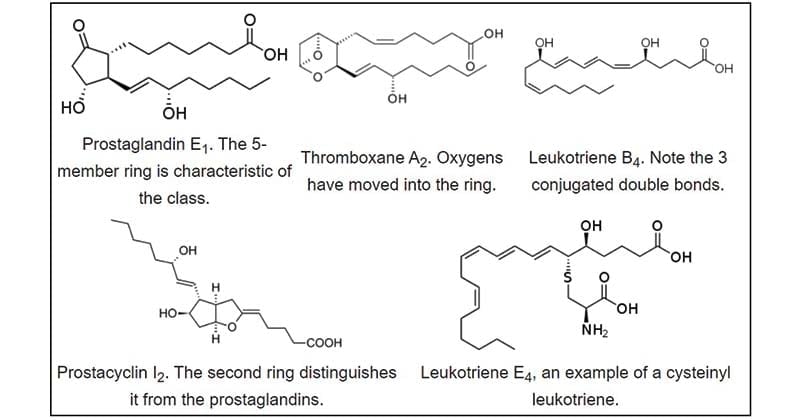
Figure: Structures of selected eicosanoids. Source: Wikipedia.
Classes of Eicosanoids
4 major classes of Eicosanoids are as following:
-
Prostaglandins (PGs)
Prostaglandins are synthesized in the prostate gland and are generally act locally at nearby tissues. They are unstable, metabolized rapidly to inactive products at the site of action. Therefore, there is no significant concentration of prostaglandins in blood. Prostaglandins have various biological functions that include stimulation of smooth muscle contraction of the uterus, the effect on the flow of blood to specific organs, inflammation, fever, pain, and the wake-sleep cycle and other biological functions.
-
Thromboxanes (TXs)
Thromboxanes are synthesized from platelets(thrombocytes) and usually have a 6-membered ring that contains an ether. Thromboxanes have a function in causing clotting of blood and decrease the flow of blood towards the clot.
-
Leukotrienes (LTs)
Leukotrienes are synthesized basically in leukocytes. The leukotrienes have 3 conjugated double bonds. These can act as strong biological signals. For instance, Leukotriene D4 causes smooth muscle contraction that lines the airways to the lung. The adverse effect of increased production of leukotrienes includes asthmatic attacks.
-
Lipoxins (LXs)
Lipoxins are formed through Lipoxygenase interactions. Lipoxins are linear eicosanoids similar to leukotrienes. They have many hydroxyl groups in the chain that is the distinguishing feature of Lipoxins. Lipoxins are generally have the potential to cause anti-inflammatory activity and its production is enhanced in response to aspirin.
Nomenclature
The nomenclature of eicosanoid includes a letter designation for the functional groups present on the ring and numbers are used for the representation of the double bonds present in the hydrocarbon chain. Prostanoids are the term used collectively for prostaglandins and thromboxanes. In the nomenclature of prostanoids, the number of carbon-carbon double bonds are represented by a subscript number. Most of the prostanoids have 2 carbon-carbon double bonds and thus called as series 2 molecules. The series 2 prostaglandins are derived from arachidonic acid and series 3 prostaglandins are synthesized from EPA. The most dominant in leukotrienes are series 4 molecules, having 4 carbon-carbon double bonds. due to the presence of four carbon-carbon double bonds.
Biological Functions
Eicosanoids have various biological functions in vertebrates. The main functions include a role in inflammation, function in reproduction, gastric secretion, and regulation of blood pressure. Other homeostatic functions by eicosanoids include regulation of vascular leakage, protection of mucosal integrity of the stomach, and regulation of aggregation of platelets.
For instance, TxA2, the COX1-derived metabolite, has an important role in platelet aggregation. On the contrary, PGI2, COX2- derived, promotes vasodilation, and cause inhibition of platelet aggregation.
Synthesis of Eicosanoids
Primary precursor
The primary precursor of the prostaglandins and other related eicosanoids is the Arachidonic acid, containing 20 carbons. Arachidonic acid is primarily the constituent of cell membrane phospholipids, mainly found in phosphatidylinositol and in other complex forms of lipid. The release of free arachidonic acid, associated with phospholipids, is performed mainly by the activity of an enzyme called phospholipase A2 and other acyl hydrolases also play some role. The release of free arachidonic acid is under controlled of hormones and other stimuli.
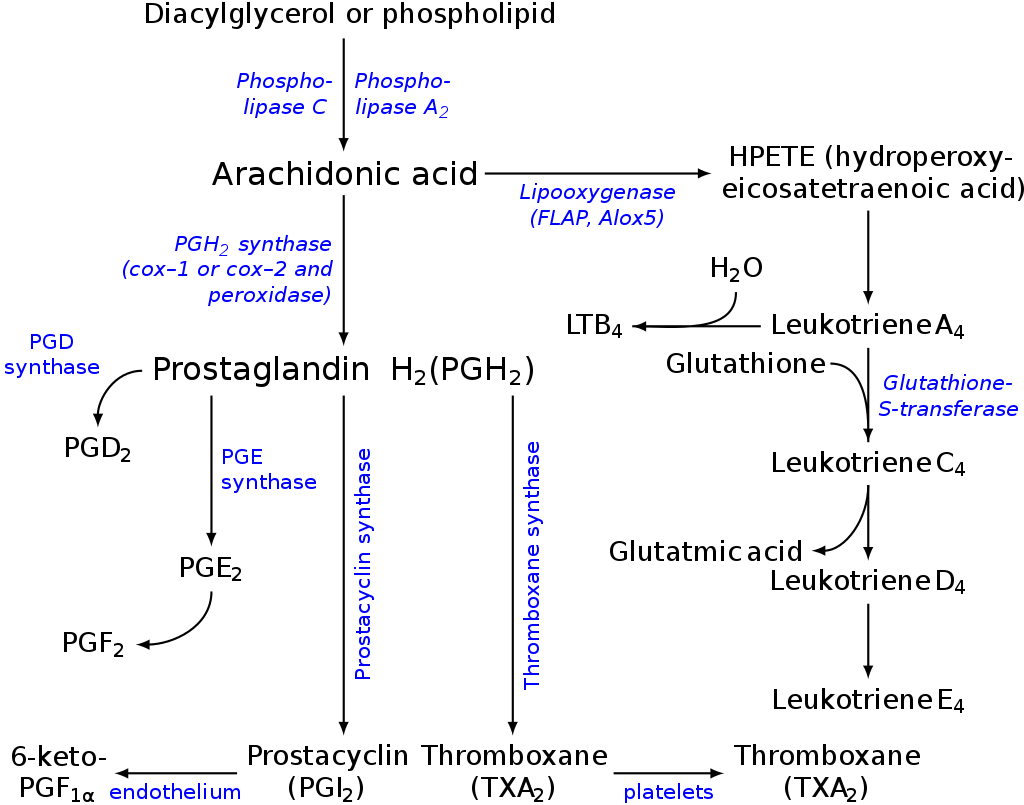
Figure: Pathways in the biosynthesis of eicosanoids from arachidonic acid: there are parallel paths from EPA & DGLA. Source: Wikipedia.
Pathways of eicosanoid synthesis
There are two major pathways involved in eicosanoid synthesis:
1.Cyclooxygenase pathway:
All eicosanoids with ring structures (example: prostaglandins and thromboxanes) are formed by the cyclooxygenase pathway. In this pathway, 2 isoforms of the cyclooxygenase enzyme, cyclooxygenase-1(COX-1), and cyclooxygenase-2 (COX-2) are involved.
Cyclooxygenase-1 (COX-1):
COX-1 involves in the production of prostanoids under physiologic conditions.COX-1 involves in the regulation of physiological cellular functions, like vascular homeostasis, kidney functions, the formation of platelet aggregates, and other normal processes.
Cyclooxygenase-2 (COX-2):
COX-2 causes an increase in the production of prostanoids in the presence of inflammation and at sites of chronic disease. However, COX-2 is expressed constitutively in tissues of the brain, bone, and kidney.
2. Lipoxygenase pathway: Leukotrienes and lipoxins are synthesized by the lipoxygenase pathway. Lipoxygenases are the enzymes that act on arachidonic acid to form unstable peroxide derivatives (5-HPETE, 12-HPETE, and 15-HPETE), then these unstable compounds can be converted to hydroxylated derivatives (the HETEs) or to lipoxins or leukotrienes. For the treatment of asthma, anti leukotriene drugs can be used.
References
- Lehninger Principles of Biochemistry Seventh Edition, 2017 David L. Nelson; Michael M. Cox.
- Lippincott’s Illustrated Reviews: Pharmacology 5th edition
- Dennis, E. A., & Norris, P. C. (2015). Eicosanoid storm in infection and inflammation. Nature Reviews Immunology, 15(8), 511-523.
- https://www.lipidhome.co.uk/lipids/fa-eic/eicintro/index.html
Sources
- 3% – https://themedicalbiochemistrypage.org/eicosanoids.php
- 1% – https://www.youtube.com/watch?v=vybF1ZACOyw
- 1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/polyunsaturated-fatty-acid
- 1% – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/lipoxygenase
- 1% – https://www.researchgate.net/publication/308902740_Leukotrienes
- 1% – https://www.physiology.org/doi/abs/10.1152/physiol.00034.2008
- 1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4516524/
- 1% – https://www.atsjournals.org/doi/pdf/10.1164/ajrccm.161.supplement_1.ltta-15
- 1% – https://www.annualreviews.org/doi/10.1146/annurev.pharmtox.45.120403.100045
- 1% – https://study.com/academy/lesson/eicosanoids-definition-function-types-effects.html
- 1% – https://pubchem.ncbi.nlm.nih.gov/compound/epoprostenol
- 1% – http://www.medicinebau.com/uploads/7/9/0/4/79048958/chapter_36.pdf
- 1% – http://themedicalbiochemistrypage.org/eicosanoids.html
- 1% – http://bio.ijs.si/~krizaj/group/Predavanja%202011/Biochemistry%20Lipids%20Lipoproteins%20and%20Membranes/13.pdf
- <1% – https://www.physiology.org/doi/full/10.1152/physiol.00036.2010
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3195913/

Really very easy, comprehensive and usefull notes