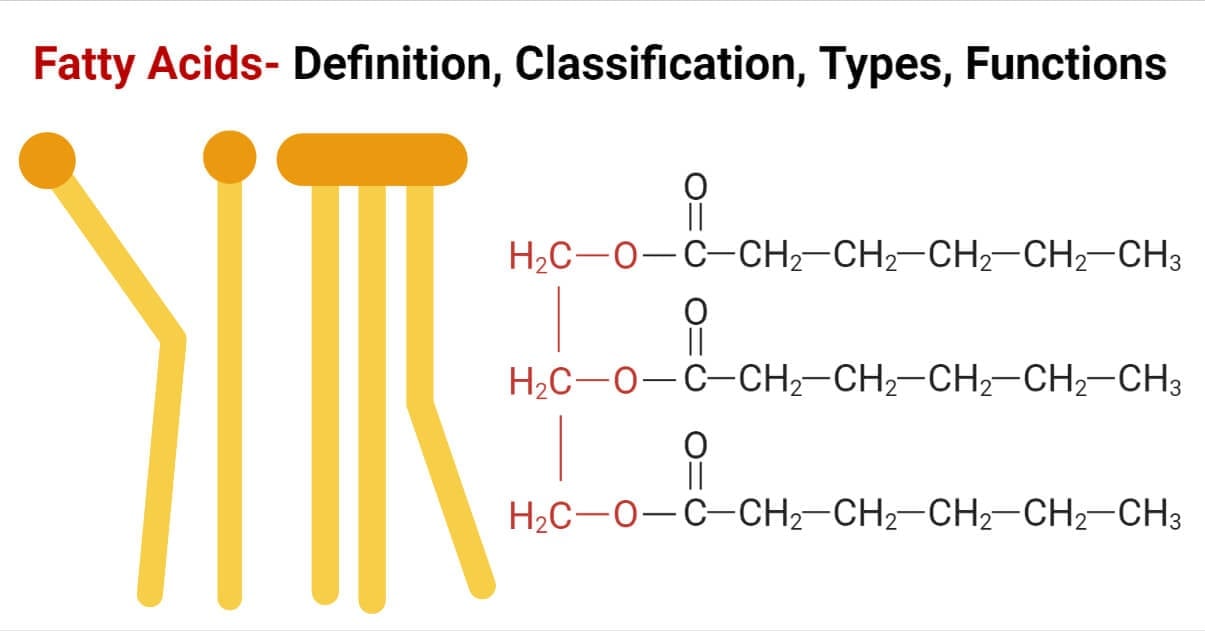
Fats are a type of simple lipids, which are esters of fatty acid and glycerol, and remain solid at room temperature.
A fatty acid is a component of lipids, which are made up of carboxylic acids and a hydrocarbon side chain.
(Fatty Acid + Glycerol = Fats)
Interesting Science Videos
What are Fatty Acids?
- Fatty acids consist of a hydrophobic hydrocarbon chain with a terminal carboxylic acid hence it is also termed aliphatic carboxylic acids.
- They occur in esterified form as well as a free fatty acid form.
- It is found in fats, oils, and other lipids In the esterified form.
- As free fatty acids (FFA) it is found unesterified in the plasma.
- Most of the fatty acids found in natural lipids contain even carbon atoms (14C-20C). This is because the biosynthesis of fatty acids occurs with the addition of 2 carbon units.
- Palmitic acid (16C) and stearic acid (18C) are most commonly found.
Classification of Fatty Acids
Saturated, Unsaturated fatty acids and Eicosanoids
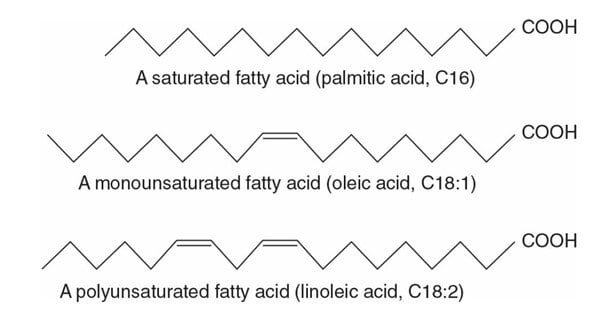
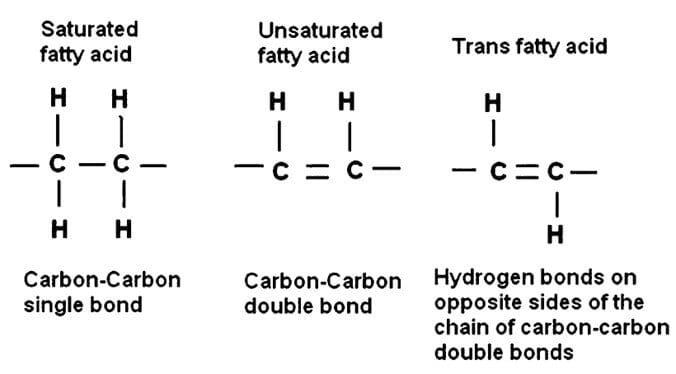
- If the hydrocarbon chain of the fatty acid contains no double bond it is termed saturated fatty acids since there is no room for any more bonding.
- If the hydrocarbon chain of the fatty acid contains one or more double bonds, it is termed unsaturated fatty acid
- Eicosanoids are derived from 20 carbon, polyenoic fatty acids, which are made up of prostanoids, leukotrienes, and lipoxins.
- Fatty acids with one double bond are called monounsaturated fatty acid
- Fatty acids with two or more double bonds are called polyunsaturated fatty acids (PUFA)
- In unsaturated fatty acids, the double bonds are generally spaced at three carbon intervals.
- Most naturally occurring unsaturated fatty acids have the cis configuration of double bonds
- The addition of a double bond decreases the melting temperature (Tm) of the fatty acid.
Length of hydrocarbon chain
- Short-chain fatty acid contains less than 6 carbons in the hydrocarbon chain
- It is termed medium-chain fatty acid if it contains 8- 14 carbons
- Long-chain fatty acid contains 16 -24 carbons in the hydrocarbon chain
- The melting temperature (Tm) of the fatty acid increases with the addition of carbon atoms to the hydrocarbon chain.
Essential and non-essential fatty acids
- Essential fatty acids (EFA) are not synthesized by the body hence they must be obtained from dietary sources
- Linoleic acid is a precursor of Omega-6 arachidonic acid.
- α-linolenic acid, a precursor of Omega-3 fatty acid
- Non-essential fatty acids are synthesized by a healthy body as long it gets enough essential fatty acids.
- However, research shows the additional health benefits of direct consumption of non-essential fatty acids as well.
Nomenclature of Fatty Acids
- The systematic nomenclature of the fatty acid is based on the hydrocarbon it is derived from.
- The names of the saturated fatty acids end with a suffix -anoic (e.g., octanoic acid), whereas an unsaturated fatty acid’s name ends with a suffix -enoic (e.g., octadecenoic acid).
- The numbering of carbon atoms begins from its carboxyl carbon, hence the carboxy carbon is given the number 1. Adjacent carbon atoms are numbered 2, 3, 4 so on. The second, third, and fourth carbons are also referred to as α, β, and γ.
- The terminal carbon atom on the other end containing the methyl group is referred to as Omega (ω) carbon. Carbon atoms are alternatively numbered from the ω carbon side as ω1, ω2, ω3, ω4, etc.
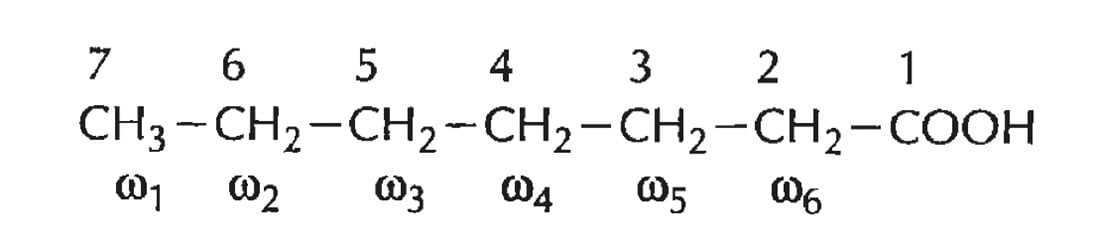
Trans Fats
- Trans fats are unsaturated fatty acids in which the double bond is arranged in a trans configuration.
- Most naturally occurring unsaturated fatty acids in the body have double bonds with cis configuration.
- Trans fatty acids arise primarily as an industrial source when edible oil containing unsaturated fatty acid is partially hydrogenated. Additionally, the unsaturated fatty acid in the rumen of ruminants like cows, goats, and sheep is transformed by bacteria into trans fat.
- Trans fatty acids also arise when cooking oil is heated to a very high temperature or when the cooking oil is repeatedly heated many times.
- Consumption of trans fats is associated with cardiovascular diseases, diabetes mellites, colon cancer, etc.
Role of Fatty Acids
- Fatty acid as a modulator of membrane properties:
- Prokaryotes, like bacteria, alter the fatty acid composition to maintain membrane fluidity in response to temperature changes.
- The eukaryotic cell membrane is made up of a phospholipid bilayer, which controls the transport of molecules to and for, regulates cellular communications, and protects the cell from the surrounding environment.
- Energy supply and storage material:
- Fatty acids are used to produce neutral storage lipids like triglycerols (TAG), Polyhydroxyalkanoates (PHA), and wax esters.
- Omega 3 PUFA (Polyunsaturated fatty acids) is required for normal growth and functioning of the brain and retina.
- Docosahexaenoic acid (DHA) acts as a neurotrophic moderator, modulates synaptic activity, involved in anti-inflammatory signaling.
- Biomarkers of organism:
- A certain fatty acid is specific to a member of a taxon of a bacterium as well as a eukaryotic cell, additionally, fatty acids have structural diversity.
- Hence the presence of certain fatty acids and their ratio is a good biomarker.
- Prokaryotes can be identified by their fatty acid fingerprint.
- The fatty acid markers are used to track energy fluxes in food webs.
References
- Dhaka et al. (2011). Trans fats—sources, health risks, and alternative approach – A review. J Food Sci Technol,48(5),534–541.
- Carla et al. (2018). The Various Roles of Fatty Acids. Molecules. 23, 2583.
- Ferrier, D., 2014. Lippincott’s Illustrated Reviews: Biochemistry. 6th ed. 351 West Camden Street Two Commerce Square; 2001 Market Street Baltimore, MD 21201 Philadelphia, PA 19103: Walter Kluwer, pp.340-344.
- Rodwell, V., Bender, D., Botham, K., Kennelly, P., and Weil, P., 2018. Harper’s illustrated biochemistry. 31st ed. United stated: McGraw-Hill, pp.484-494.
- Sathyanarayana, U.,Chakrapani, U., 2006. Biochemistry. 3rd ed. Kolkata, India: Books and allied (P), Ltd, pp.28-34.
