Alpha-actinin is an actin-binding protein with F-actin (filamentous) binding activity but lacks G-actin (Globular) binding activity.
They are ubiquitously present in the cytoskeleton of muscle (calcium insensitive) and non-muscle cells (calcium sensitive), such as neurons and fibroblasts. It is majorly responsible for the structural component of the cytoskeleton that aids in the binding and organizing of actin filaments in cells. They belong to a larger superfamily of proteins called the spectrin and are distributed in different parts of cells depending on the type of tissue they are present in.
Their function includes stabilizing the contractile muscle apparatus, modulating several receptor activities, and acting as a scaffold connecting different proteins in the cytoskeleton used in cell signaling pathways. The presence of alpha-actinin at multiple subcellular locations, such as cell protrusions, cell-matrix contact sites, and stress fiber-dense regions in both muscle and non-muscle cells, indicates the importance of this protein in linking cytoskeleton components to different proteins present in cells.
Their existence as isoforms has been persistent genetically and biochemically in mammals and species, including protists, invertebrates, and birds. Mammalian cells exhibit the highest level of diversity, with four diverse alpha-actinin-encoding genes producing at least six different protein products, each having its particular tissue type and expression pattern.
Interesting Science Videos
Actin-Parent Molecule
Actin molecule is a protein that is most abundantly present in eukaryotic cells. They are highly conserved proteins throughout evolution and are involved in more protein-protein interaction than other researched proteins.
- It is an important contributor to muscle contraction apparatus. Actin filaments are made up of repetitive units of actin protein in two forms – monomeric G-actin (globular) and polymeric F-actin (filamentous).
- They serve as the building block of the cytoskeleton that facilitates cellular movements, ranging from cell shape maintenance to cell motility and regulation of transcription.
- Actin filaments and myosin are activated during muscle contraction, and tropomyosin proteins regulate their fusion during contraction. They regulate the dynamic structures of various cell types via cellular and subcellular movements.
Alpha-Actinin Functions
Their principle function involves the cross-linking of actin filaments and other cytoskeleton components affecting the cohesiveness and mechanics of cellular movement. The scaffold provides actin filaments with stability and acts as a bridge for cytoskeleton integration into signalling pathways. Alpha-actinin is thought to interact with approximately 30 cellular components, and their isoforms appear to be active in nuclear events. They are highly active in polarised cells, with extensive actin polymerization aiding the migration and adhesion of cells. They are the primary cross-linkers in the stress fibres and are abundantly present in dendritic spines, leading to focal adhesion and neuritic growth. Their interaction does localization of this protein to cellular compartments such as plasma membranes with transmembrane receptors and membrane lipids.
Alpha-Actinin Types
Four alpha-actin types can be observed that regulate the cytoskeleton activity in muscle and non-muscle cells.
- ACTN1: Encoded by the ACTN1 gene and responsible for Z-lines attachment to actin myofibrils in skeletal muscle cells. In smooth muscle cells, ACTN1 attaches dense bodies to the actin filaments
- ACTN2: Encoded by the ACTN2 gene in humans, composed of 894 amino acids and weighs approximately 103.8 kDa, expressed in skeletal and smooth muscle cells. They anchor filamentous actin fragments to Z-disks mediated by phospholipids.
- ACTN3: Encoded by ACTN3 gene, also known as Sprinter gene on chromosome 11, expressed exclusively in type II muscle fibres. They are involved in enhanced athletic performance and muscle injury recovery in the elite sports individual.
- ACTN4: Encoded by the ACTN4 gene and involved in the cytoskeleton’s metastatic regulations. Mutations in this actinin lead to kidney glomerulosclerosis.
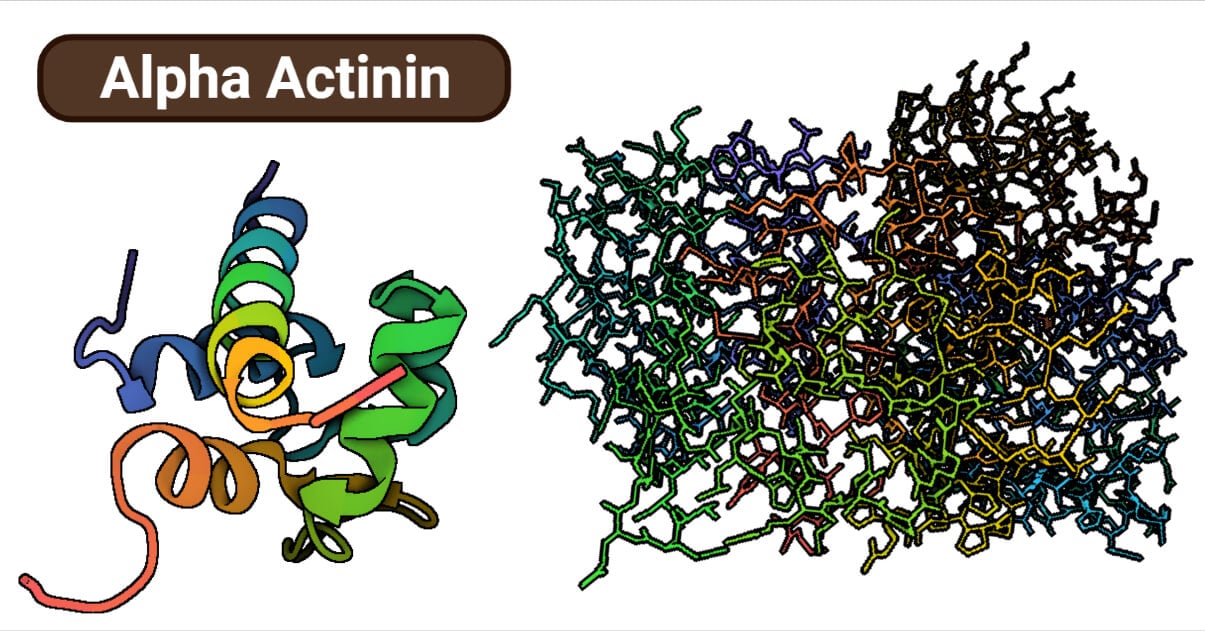
Alpha-Actinin Structure
Alpha-actinin is an anti-parallel homodimer playing a central role in cross-linking actin filaments in the cells. All spectrin superfamilies, including the alpha-actinin, have an N-terminal Actin binding domain (ABD), spectrin repeats (SR) and C-terminal calmodulin-like domain (CaM) as a distinctive feature.
- The ABD region comprises two calponin homology (CH) domains.
- The spectrin repeats (SR) are responsible for determining the length and flexibility of the actin-binding protein and the type of actin filament cross-links. Since the SR region is cylindrical, it constitutes the rod domain, and ABD and four SR constitute the functional core rod in the anti-parallel dimer of alpha-actinin.
- The C-terminal CaM domain is at the end of the central rod, opposite the ABD. It has two pairs of EF-hand motifs (EF1/2 and EF3/4) which has a regulatory function in the binding protein. Some actinins bind to the calcium, which prevents the actin-binding at high calcium concentrations in muscle and non-muscle cells, thus enabling cross-linking of actin filaments into bundles.
N-terminal Actin Binding Domain (ABD)
ABD of alpha-actinins is the most conserved domain within the spectrin family proteins throughout the evolutionary conservation of actin, its binding partner. ABD is composed of two tandem pairs of Calponin Homology (CH) made up of 100 amino acids in length, arranged in four alpha helices. It is of two types – type 1 (CH1) and type 2 (CH2), differing in amino acid sequence. In an unbound state, it is considered that ABD is present as a closed conformation state.
- The core of the actin-binding domain comprises four principle helices named A, C, E and G, present in each individual calponin (CH) domain.
- Helices A and E are present outwardly, while the helices C and G are sandwiched between them.
- Three minor helices can be identified in the spectrin family ABD but only two helices, B and F, are seen in alpha-actinin ABD.
- CH1 And CH2 domain interaction is semipolar, with some hydrophobic parts and some polar parts stabilizing the interface.
According to studies, the surfaces of the two CH domains complement one another, much like the interfaces in permanent complexes and complexes containing enzyme inhibitors. It is conferred that CH1 and CH2 together lead to higher affinity actin binding. It is observed that the CH2 tandem domain alone is not sufficient for cross-linking actin filaments, while CH1 has a lower affinity to bind.
Rod Domain-Multiple Spectrin Repeats
It is considered the least conserved domain throughout evolution owing to the diversity in the number of spectrin repeats of the alpha-actinin protein in different organisms. They are involved in determining the length and flexibility of the protein and the nature of actin filament cross-links. The central rod region typically has four consecutive spectrin repeats in all known vertebrate alpha-actinin.
- Organisms like protozoans, fungi and parasites have one, two and five repeats, respectively.
- The four repeats in vertebrates are said to originate from a precursor protein with one repeat through two intragenic duplication events.
Each spectrin repeat has a conformation of a helix-coiled bundle as a three-dimensional structure.
- An anti-parallel homodimer containing the alpha-Actinin rod domain has an overall length of 240 A and a breadth of 40–50 A.
- The repeating units are joined by short, stiff helical linkers that give the subunit and the dimer structural rigidity, a property necessary for its main function—the bundling of actin filaments.
The spectrin repeats have sequences largely of basic nature owing to its natural tendency for helical secondary structure.
- These repeats are typically around 80 amino acids in length and are characterized by specific sequence motifs, such as the consensus sequence Asp-Pro-Gly-X-X-Pro.
- However, the amino acid composition of spectrin repeats differs slightly between various spectrin isoforms and organisms.
- In general, spectrin repetitions are rich in hydrophobic amino acids like leucine, isoleucine, and valine, which are known to be crucial for the stability of the protein.
- Additionally, they have significant concentrations of proline and glycine. These two amino acids are frequently found in structural proteins and are thought to be crucial for preserving the flexibility and form of the protein.
C-terminal Calmodulin-like (CaM) Domain
A calcium-binding protein that plays a crucial role in intracellular signalling. The CaM-like domain is often found at the C-terminus (the end of the protein). It is responsible for binding to CaM and transmitting signals responding to changes in intracellular calcium levels. Four alpha helices or two pairs of EF motifs (EF1/2 and EF3/4) are bundled together and folded into a small compact globular structure to form the CaM domain.
- EF-hands are used for binding calcium inside of cells are Helix-loop-helix motifs. EF-hands typically exist in pairs, creating a globular domain that can coordinate up to two calcium ions.
- Calcium binding causes the globular domain to alter significantly from a closed to an open state, rearranging the a-helices and exposing hydrophobic residues to the protein’s surface, allowing the protein to interact with particular targets.
- EF-hand motifs show functional divergence amongst alpha-actinin isoforms (muscle and non-muscle).
- Muscle isoforms are regulated by binding the phospholipid phosphatidylinositol 4,5-biphosphate (PiP2). The EF-hands of a-non-muscle (such as fibroblasts and neurons) actinin’s isoforms bind calcium, exerting regulatory control over the neighbouring ABD’s actin-binding activity that of other proteins.
Due to changes in the residues involved in calcium coordination, muscle isoforms have lost their capacity to bind calcium. While the calcium-insensitive isoforms of alpha-actinin emerged with the development of muscle tissue, where actin-binding regulation should be independent of calcium flow, the original gene encoding alpha-actinin encoded a calcium-sensitive isoform during the evolution of a-actinin.
Regulation by Different Mechanisms
Numerous processes control alpha-actin activity, such as post-transcriptional alterations, protein-protein interactions, and adjustments to intracellular signaling pathways. For instance, phosphorylation with proteins, like talin and vinculin, can change the activity of alpha actinins and regulate how they bind to actin filaments. Alpha actinins can also be controlled by variations in intracellular calcium levels, which may impact how alpha actinins bind to actin filaments. Two of the regulatory events are discussed below:
Phosphorylation
Phosphorylation is adding a phosphate group to a protein, which can alter its activity and interaction with other proteins.
- Phosphorylation of specific sites in alpha-actinin can lead to its activation by promoting its association with actin filaments.
- In contrast, other phosphorylation sites can lead to its inhibition by decreasing its association with actin filaments.
- The kinase enzymes that catalyze the phosphorylation reaction and the specific sites that are phosphorylated are key factors in determining the effects of phosphorylation on alpha-actinin activity.
An autosomal dominant disease FSGS (Focal Segmental Glomerulosclerosis) caused due to the natural mutation of amino acid sequence in ABD of alpha-actinin 4 (ACTN4) can be modulated by phosphorylation. When tyrosine 4 and tyrosine 31 are phosphorylated via growth factors in a hierarchical order, it is observed that there is a significant decrease in the actin-binding activity of ACTN4. Thus preventing excessive cellular aggregation and facilitating cell motility.
Proteolytic Cleavage
Regulation of alpha-actinin protein by proteolytic cleavage mechanism helps to stabilize and organize actin filaments in cells. Proteolytic cleavage leads to the formation of smaller alpha-actin fragments that represent different functions and activities than the whole protein. The effects of proteolytic cleavage on alpha-actinin activity are largely determined by the specific proteases that mediate the cleavage and the particular sites that are cleaved.
- Alpha actinin 4 (ACTN4) is responsible for the arrangement of actin bundles in cells for focal adhesion and cell motility.
- ACTN4 contains a calpain cleavage site in the N-terminal and C-terminal regions. Cleavage at N-terminal tyr13 and Gly14 of ACTN4 doesn’t affect the actin-binding activity of the protein but aids in the actin bundle arrangement involved in cell migration.
- However, cleavage at the C-terminal of ACTN4 or any other alpha-actinin can lead to a decrease in the calcium-regulated binding of actin filaments, hindering focal adhesion and cell motility.
Calpain protease has also been observed to regulate the T-cell’s alpha-actinin activity. Stimulation of CD3 and T-cell receptors activates actinin-mediated rearrangement of the cytoskeleton. Cleaved actinin enhances the spreading and cytoskeleton plasticity due to ease in the movement of smaller actinin proteins.
Conclusion
The role of alpha-actinin as a multi-tasking actin-binding crosslinking protein. They are responsible for the bundling of actin and cytoskeleton filaments and aid in the translocation of the membrane and intracellular proteins involved in signalling pathways.
References
- Sjöblom, Björn, A. Salmazo, and K. Djinović-Carugo. “α-Actinin structure and regulation.” Cellular and molecular life sciences 65 (2008): 2688-2701.
- Kelkar, Manasi, Pierre Bohec, and Guillaume Charras. “Mechanics of the cellular actin cortex: From signalling to shape change.” Current opinion in cell biology 66 (2020): 69-78.
- Alpha-actinin | MBInfo – https://www.mechanobio.info/cytoskeleton-dynamics/actin-crosslinking/alpha-actinin/
- Dominguez, Roberto, and Kenneth C. Holmes. “Actin structure and function.” Annual review of biophysics 40 (2011): 169-186.
- Actin – https://www.britannica.com/science/actin
- Ribeiro Jr, Almeida, et al. “The structure and regulation of human muscle α-actinin.” (2014).
- Calponin homology domain – https://en.wikipedia.org/wiki/Calponin_homology_domain
- Fukami, Kiyoko, et al. “alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase.” Journal of Biological Chemistry 269.2 (1994): 1518-1522.
- Shao, Hanshuang, et al. “The carboxyl tail of alpha-actinin-4 regulates its susceptibility to m-calpain and thus functions in cell migration and spreading.” The international journal of biochemistry & cell biology 45.6 (2013): 1051-1063.
- Shao, Hanshuang, et al. “Focal segmental glomerulosclerosis ACTN4 mutants binding to actin: regulation by phosphomimetic mutations.” Scientific Reports 9.1 (2019): 15517.
- MacArthur, Daniel G., and Kathryn N. North. “A gene for speed? The evolution and function of α‐actinin‐3.” Bioessays 26.7 (2004): 786-795.
- Selliah, Nithianandan, William H. Brooks, and Thomas L. Roszman. “Proteolytic cleavage of alpha-actinin by calpain in T cells stimulated with anti-CD3 monoclonal antibody.” Journal of immunology (Baltimore, Md.: 1950) 156.9 (1996): 3215-3221.
- Actinin – https://en.wikipedia.org/wiki/Actinin
- Alpha-actinin-2 – https://en.wikipedia.org/wiki/Alpha-actinin-2
- Pickering, Craig, and John Kiely. “ACTN3: more than just a gene for speed.” Frontiers in physiology 8 (2017): 1080.
- Alpha-actinin-3 – https://en.wikipedia.org/wiki/Alpha-actinin-3
- Alpha-actinin-4 – https://en.wikipedia.org/wiki/Alpha-actinin-4
