Interesting Science Videos
What is a spectrophotometer?
- A spectrophotometer is an instrument that measures the amount of light absorbed by a sample.
- Spectrophotometer techniques are mostly used to measure the concentration of solutes in solution by measuring the amount of the light that is absorbed by the solution in a cuvette placed in the spectrophotometer.
- Scientist Arnold J. Beckman and his colleagues at the National Technologies Laboratory (NTL) invented the Beckman DU spectrophotometer in 1940.
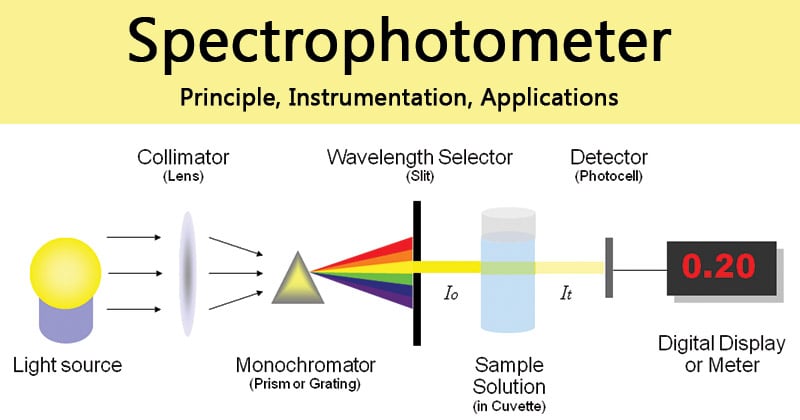
Principle of Spectrophotometer
The spectrophotometer technique is to measure light intensity as a function of wavelength. It does this by diffracting the light beam into a spectrum of wavelengths, detecting the intensities with a charge-coupled device, and displaying the results as a graph on the detector and then on the display device.
- In the spectrophotometer, a prism (or) grating is used to split the incident beam into different wavelengths.
- By suitable mechanisms, waves of specific wavelengths can be manipulated to fall on the test solution. The range of the wavelengths of the incident light can be as low as 1 to 2nm.
- The spectrophotometer is useful for measuring the absorption spectrum of a compound, that is, the absorption of light by a solution at each wavelength.
Instrumentation of Spectrophotometer
The essential components of spectrophotometer instrumentation include:
- A table and cheap radiant energy source
- Materials that can be excited to high energy states by a high voltage electric discharge (or) by electrical heating serve as excellent radiant energy sources.
- A monochromator, to break the polychromatic radiation into component wavelength (or) bands of wavelengths.
- A monochromator resolves polychromatic radiation into its individual wavelengths and isolates these wavelengths into very narrow bands.
Prisms:
- A prism disperses polychromatic light from the source into its constituent wavelengths by virtue of its ability to reflect different wavelengths to a different extent
- Two types of Prisms are usually employed in commercial instruments. Namely, 600 cornu quartz prism and 300 Littrow Prism.
Grating:
- Gratings are often used in the monochromators of spectrophotometers operating ultraviolet, visible and infrared regions.
- Transport vessels (cuvettes), to hold the sample
- Samples to be studied in the ultraviolet (or) visible region are usually glasses (or) solutions and are put in cells known as “CUVETTES”.
- Cuvettes meant for the visible region are made up of either ordinary glass (or) sometimes Quartz.
- A Photosensitive detector and an associated readout system
- Most detectors depend on the photoelectric effect. The current is then proportional to the light intensity and therefore a measure of it.
- Radiation detectors generate electronic signals which are proportional to the transmitter light.
- These signals need to be translated into a form that is easy to interpret.
- This is accomplished by using amplifiers, Ammeters, Potentiometers and Potentiometric recorders.
Applications
Some of the major applications of spectrophotometers include the following:
- Detection of concentration of substances
- Detection of impurities
- Structure elucidation of organic compounds
- Monitoring dissolved oxygen content in freshwater and marine ecosystems
- Characterization of proteins
- Detection of functional groups
- Respiratory gas analysis in hospitals
- Molecular weight determination of compounds
- The visible and UV spectrophotometer may be used to identify classes of compounds in both the pure state and in biological preparations.
References
- https://www.biochemden.com/spectrophotometer-instrumentation-principle/
- https://www.azom.com/article.aspx?ArticleID=10245
- https://web.stanford.edu/class/chem184/lectures08/Zare_Spectroscopy.pdf
- https://byjus.com/chemistry/spectrophotometer-principle/
- https://www.slideshare.net/rey216/spect

t`hanks but what is the difference between colorimeter and spectrophotometer?
Spectrophotometer – it is a instrument used to measure the how much chemical substance observe a light when pass through a solution
Colorimeter – it is a instrument used to measure the transmittance and observance of light when pass through a solution
What is the difference b/w Sample solution and reference blank? why we use reference standard in in Spectrophotometer??
It really help alot
thank you for giving this more information about specrophotometer. Actually, It very helps for answering questions me.
Interesting
Nice one
Spectrophotomer or Spectrophotometery ya dono same haiin kia
Spectrophotometer bis the device
Spectrophotometery is the technique
Thanks fr giving the sufficient knowledges about Spectrophotometer….
Please be more scientific, I guess this is not a complete explanation of principle of spectrophotometer.
Yah
Thanks