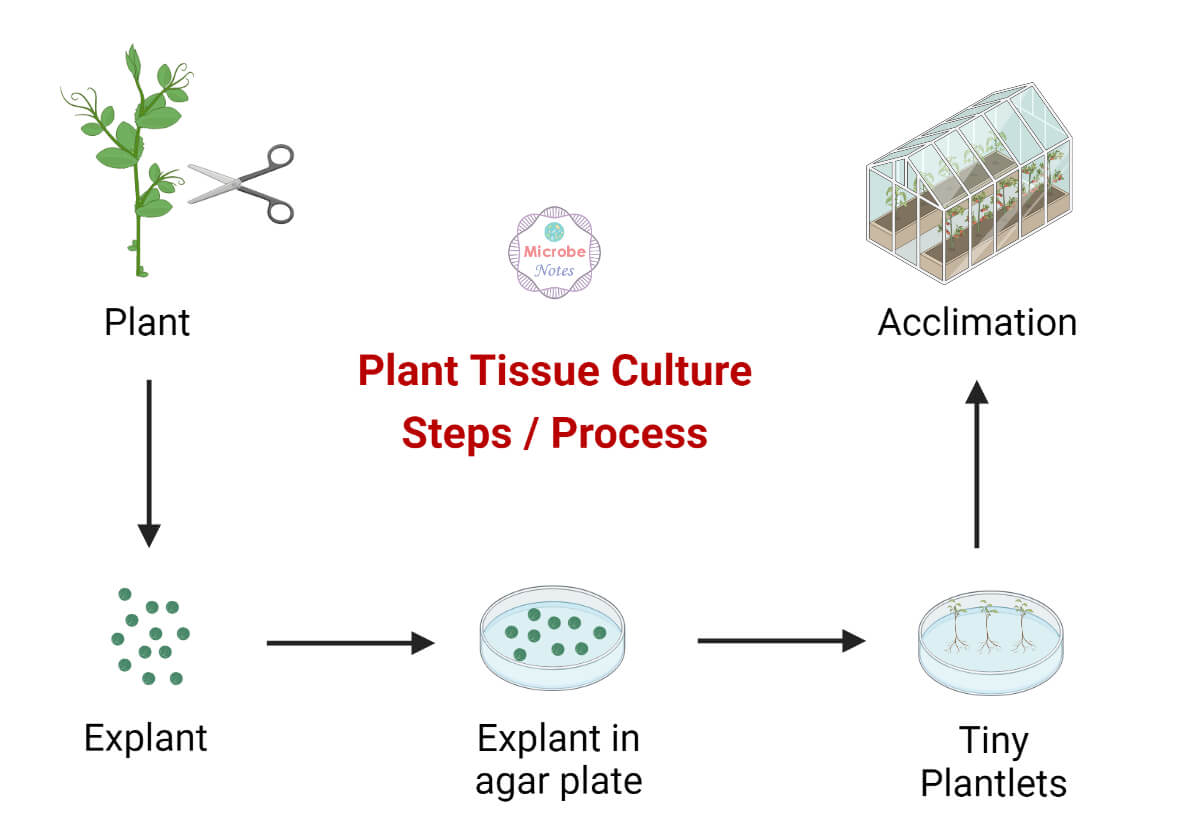
Plant tissue culture is the in-vitro aseptic culture of cells, tissues, or whole plants under controlled nutritional and environmental conditions, often to produce clones of plants.
The technique primarily relies on plant cells’ totipotency which is the capacity of a single cell to express the whole genome during cell division. The ability of cells to change their metabolism, growth, and development is just as significant and essential for the regeneration of the entire plant.
Plant tissue culture technology is being widely used for large-scale plant multiplication. In addition to being used in research, they are now essential for plant propagation, disease eradication, and the generation of secondary metabolites.

Plant Tissue Culture History
Gottlieb Haberlandt, in 1902 tried to cultivate individual palisade cells from leaves in knop’s salt solution supplemented with sucrose. The cells sustained for a month stored starch but ultimately did not divide. Despite his failure, he is considered the father of plant tissue culture since his experiment set the stage for developing tissue culture technology. Similarly, Roger J. Gautheret, a French scientist, had encouraging results with culturing cambial tissues of carrots in 1934.
Plant Tissue Culture Conditions
The choice of medium is based on the types of plant species; explants are used for culture for optimal response. All the nutrients required for a plant’s proper growth and development should be present in the plant tissue culture media. Macronutrients, micronutrients, vitamins, other organic ingredients, plant growth regulators, a carbon source, and in the case of a solid medium, a few gelling agents make up the majority of its composition. Similarly, hormone levels and culture variables like temperature, pH, light intensity, and humidity also play an important role in the success of tissue culture.
- The minerals consist of macronutrients such as nitrogen, potassium, phosphorus, calcium, magnesium, and sulfur, and micronutrients such as iron, manganese, zinc, boron, copper, molybdenum, and cobalt.
- Vitamins are necessary for the healthy growth of plant cultures. The vitamins like thiamine (vitamin B1), pyridoxine (B6), and nicotinic acid (niacin). Other vitamins such as biotin, folic acid, ascorbic acid (vitamin C), and vitamin E (tocopherol) are sometimes added to media formulations.
- Plants also require an external carbon source; sugar. The most commonly used carbon source is sucrose. Other sources used are glucose, maltose, and sorbitol.
- The pH of the culture medium remains vital as it influences the uptake of various components of the medium and regulates a wide range of biochemical reactions. Most media are adjusted to a pH of 5.2–5.8. A higher pH may be required for certain cultures.
Plant Tissue Culture Media
- The most popular medium for in vitro vegetative propagation of various plants is Murashige and Skoog medium (MS medium). For culturing, either a solid or liquid medium can be employed.
- McCown’s woody plant medium (WPM) has been widely used for tree tissue culture.
- Knudson’s medium is used for orchid tissue culture and fern tissue culture.
Plant Tissue Culture Growth regulators
- Plant growth regulators (PGRs) are crucial for determining the development of plant cells and tissues in a culture medium.
- The most commonly used plant growth regulators are auxins, cytokinins, and gibberellins.
- The high auxin concentration often favors the development of roots. The most commonly used auxins are IAA (indoleacetic acid), IBA (indolebutyric acid), NAA (naphthaleneacetic acid), and 2,4-D (2,4 dichlorophenoxyacetic acid).
- Cytokinins promote cell division and shoot growth. The most commonly used cytokinins are BAP (benzylaminopurine), zeatin, Isopentenyl adenine (2-ip), and kinetin. Cytokinins are generally dissolved in dilute HCl or NaOH.
- A clump of undifferentiated cells called a callus develops when auxin and cytokinin levels are balanced.
Plant Tissue Culture Vessels
- Another critical aspect in plant tissue cultures is the management of the gaseous hormone ethylene. In closed culture vessels used for in vitro plant growth, ethylene builds up and is often detrimental to the cultures. The addition of ethylene biosynthetic inhibitors such as silver nitrate, AVG (aminoethoxyvinylglycine), and silver thiosulphate have been shown to increase the formation of shoots.
- Cultures are grown in walk-in growth rooms or growth chambers. Humidity, light, and temperature must be controlled for the proper growth of cultures.
- A 16-hour light photoperiod is optimal for tissue cultures, and a temperature of 22 – 25⁰C is used in most laboratories.
- Cool white fluorescent lamps also supply a light intensity of 25–50 µmol m-2 s-1.
- Relative humidity of 50–60% is maintained in the growth chambers. Some cultures are also incubated in the dark.
- Cultures can be cultivated in various containers, including test tubes, flasks, Petri dishes, and bottles.
Sterility
- The preservation of a sterile environment is necessary for effective tissue culture. The laminar flow hood is used for all tissue culture work. A dust filter and a high-efficiency particulate air (HEPA) filter are used in the laminar flow hood to filter the air. The hood must be kept spotless, which can be accomplished by wiping it with alcohol that contains 70% of the alcohol.
- The surfaces of plant tissues naturally contain a variety of bacteria and fungi. Before tissue culture, it is crucial to thoroughly clean the explant since contaminants can proliferate in the culture media. They also compete with the plant tissue for nutrients, depriving it of those nutrients. Bacteria and fungi can rapidly surpass plant tissues and destroy them.
- Explants are commonly surface-sterilized using sodium hypochlorite, ethanol, and fungicides when using field-grown tissues.
- The type of tissue usually decides the time of sterilization. Leaf tissue requires a shorter sterilization time than seeds with a hard seed coat.
Plant Tissue Culture Types
Callus Culture
A callus is an unorganized mass of cells that develops when cells are wounded. When the explant is cultivated on media that promote the development of undifferentiated cells, a callus is formed. The majority of callus cells are formed with the aid of auxins and cytokinins. Using plant growth hormones, callus can multiply continuously or be directed to develop organs or somatic embryos.
Cell Suspension Culture
Small fragments of loose friable callus can be cultured as cell suspension cultures in a liquid medium. Cell suspensions can be maintained as batch cultures grown in flasks for long periods. A portion of callus tissue can be transferred into a liquid medium, and when subjected to continuous shaking, single-cell cultures and suspension cultures can be cultivated from callus cultures. The growth rate of the suspension-cultured cells is generally higher than that of the solid culture.
Anther/Microspore Culture
The culture of anthers or isolated microspores to produce haploid plants is known as anther or microspore culture. Embryos can be produced via a callus phase or be a direct recapitulation of the developmental stages characteristic of zygotic embryos. Compared to traditional breeding methods, microspore culture enables the creation of homozygous plants in a very short time. These homozygous plants are useful tools in plant breeding and genetic studies.
Protoplast Culture
Protoplasts contain all the components of a plant cell except for the cell wall. Protoplasts can be used to create somatic hybrids and regenerate whole plants from a single cell. Cell walls of explant can be removed either mechanically or enzymatically. Protoplasts can be cultured either in liquid or solid medium. Protoplasts embedded in an alginate matrix and then cultured on a solid medium have better success rates of regeneration. Although protoplasts appear to be a very appealing method for regenerating plants and transferring genes, they are extremely delicate.
Embryo Culture
It is a technique in which isolated embryos from immature ovules or seeds are cultured in vitro. For species whose seeds are dormant, resistant, or prematurely sterile, embryo culture has been used as a helpful tool for direct regeneration. In plant breeding programs, embryo culture goes hand in hand with in vitro control of pollination and fertilization to ensure hybrid production. In addition, direct somatic embryos and embryogenic calluses can be produced from immature embryos.
Meristem Culture
Using apical meristem tips, it is possible to produce disease-free plants. This technique can be referred to as meristem culture, meristem tip culture, or shoot tip culture, depending on the actual explant used. Plant apical meristems make good explants for the cultivation of virus-free plants. Hence, this method is usually used to eliminate viruses in many species.
Regeneration Methods of Plants in Culture
It includes two methods:
- Organogenesis
- Somatic Embryogenesis
Organogenesis
In plant tissue culture, it refers to the formation of either shoot or root. The equilibrium of auxin and cytokinin and the tissue’s capacity to react to phytohormones during culture are key factors in in-vitro organogenesis. In-vitro organogenesis can be of two types:
- Indirect organogenesis
- Direct organogenesis
- Indirect organogenesis involves the formation of organs indirectly via a callus phase. For the production of transgenic plants, induction of plants through a callus phase has been used. Either the callus is transformed, plants are regenerated, or the primary explant is transformed, and the callus is formed, and then shoots are cultivated from the explant. It is more important for transgenic plant production.
- Direct organogenesis involves direct bud or shoots formation from the tissue without a callus stage. Plants are usually propagated by direct organogenesis for improved multiplication rates and production of transgenic plants but mainly for clonal propagation.
Somatic Embryogenesis
Somatic embryogenesis is a nonsexual developmental process that produces a bipolar embryo with a closed vascular system from the somatic tissues of a plant. It has become one of the most powerful techniques in plant tissue culture for mass clonal propagation. Somatic embryogenesis may occur directly or via a callus phase. For clonal propagation, direct somatic embryogenesis is preferred since there is less chance of somaclonal mutation.
Indirect somatic embryogenesis is usually used in the selection of desired somaclonal variants and for the production of transgenic plants.
Encapsulated somatic embryos are known as synthetic seeds. Synthetic seeds have multiple advantages. They are easy to handle, they can potentially be stored for a long time, and there is potential for scaleup and low cost of production.
Rooting of shoots
The success of acclimatization of a plantlet greatly depends on root system production. Rooting of shoots can be achieved in vitro or ex-vitro.
Ex vitro rooting involves pretreating the shoots with phenols or auxins and then planting them directly in soil under high humidity, which significantly lowers the cost of manufacturing. This technique also allows simultaneous acclimation of the rooted shoots.
In vitro rooting consists of rooting the plants in axenic conditions. Despite the cost factor, in vitro rooting is still a common practice in many plant species.
Several factors are known to affect rooting. The most important factor is the action of endogenous and exogenous auxins. Phenolic compounds are also known to have a stimulatory effect on rooting. Phloroglucinol, a root promoter, is reported effective in root development. Catechol, a strong reducing agent, has been reported to regulate IAA oxidation.
Acclimation / Acclimatization
Once plants are generated by tissue culture, they have to be transferred to the greenhouse or field. This requires that the plants be hardened-off before transfer to the field. To reduce water loss during acclimatization, plants are initially transferred to a greenhouse or growth chamber. The relative humidity outside the vessels is often significantly lower than the humidity inside the vessels. Once the plants are acclimatized under greenhouse conditions, they are ready for transfer to the field.
Advantages of Plant Tissue Culture
- Totipotency, nutrition, metabolism, division, differentiation, and preservation of plant
cells. - Morphogenesis and plant regeneration from individual cells or tissues through organogenesis or somatic embryogenesis.
- Variations were generated through in vitro culture.
- Evolution of haploids through anther and pollen culture, including ovule culture.
- Wide hybridization programs through ovule, ovary, and embryo cultures to overcome both pre-zygotic and post-zygotic sterility mechanisms.
- Micropropagation of plant materials.
- In vitro selection of mutants tolerant to biotic and abiotic stresses.
- In vitro culture and secondary metabolite biosynthesis.
- Plant genetic engineering using DNA transfer and in vitro culture techniques.
Disadvantages of Plant Tissue Culture
- Labor-intensive and expensive process.
- Vulnerable to many environmental factors.
Reference
- Cardoza, V. (2008). Tissue Culture: The Manipulation of Plant Development. In N. C. J. Stewart (Ed.), Plant Biotechnology and Genetics (pp. 113–128). John Wiley & Sons, Inc.
- Hussain, A., Qarshi, I. A., Nazir, H., & Ullah, I. (2012). Plant Tissue Culture: Current Status and Opportunities. Intech Open. https://www.intechopen.com/chapters/40180
- ICAR. (2022). Principles of Plant Biotechnology. https://pravara.in/wp-content/themes/twentyseventeen/essentials/pdf/elearn/Principles-of-Plant-Biotechnology.pdf
