The movement of nitrogen between the atmosphere, biosphere, and geosphere in different forms is called the nitrogen cycle and is one of the major biogeochemical cycles.
- It may also be considered as the movement of nitrogen through the food chain from simple inorganic compounds, mainly ammonia, to complex organic compounds.
- This complex cycle involves bacteria, plants, and animals.
- All organisms can convert ammonia (NH3) to organic nitrogen compounds that are compounds containing C–N bonds. However, only a few microorganisms can synthesize ammonia from nitrogen gas (N2).
- Although N2 gas makes up about 80% of the earth’s atmosphere, it is a chemically unreactive compound and thus needs to be changed in order to be utilized by living beings.
- Within the biosphere, there is a balance between total inorganic and total organic forms of nitrogen.
- The conversion of organic to inorganic nitrogen comes about through catabolism, de-nitrification, and decay.
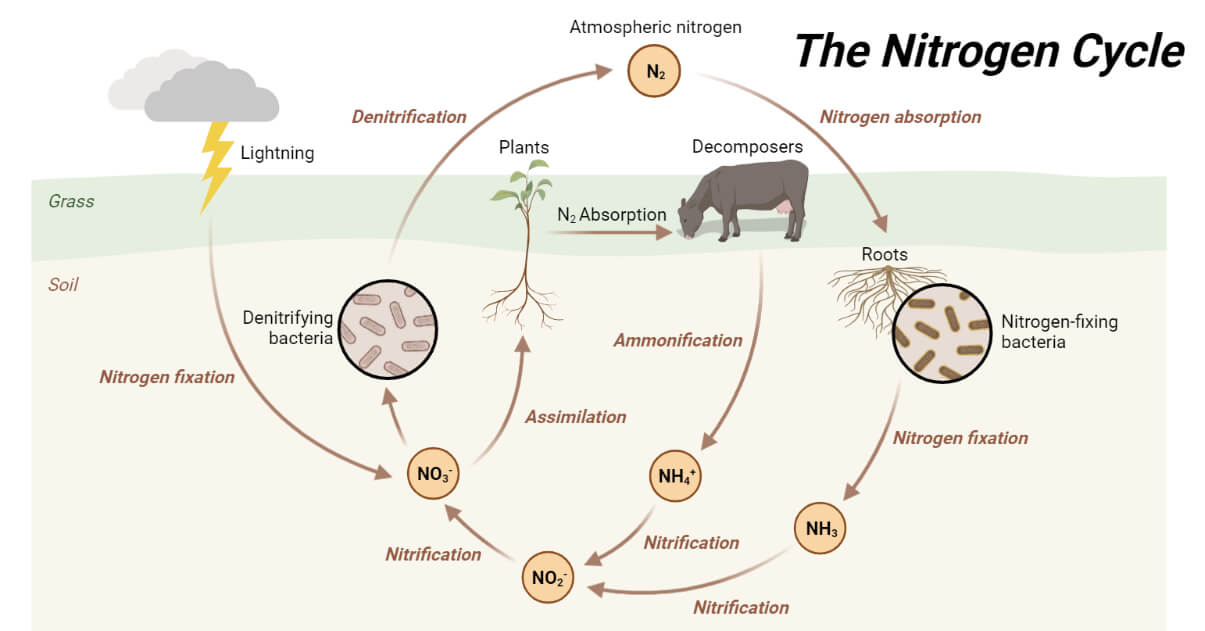
Steps of Nitrogen Cycle
1. Nitrogen Fixation
The first stage in the nitrogen cycle is the reduction of N2 gas to ammonia, a process called nitrogen fixation.
- The process of converting atmospheric N2 gas into ammonia is carried out by only a few microorganisms, termed diazotrophs which have an enzyme called “nitrogenase” that combines nitrogen atoms with hydrogen atoms.
- These are some free-living soil bacteria such as Klebsiella and Azotobacter, cyanobacteria (blue-green algae), and the symbiotic bacteria (mainly Rhizobium).
- The amount of N2 fixed by these diazatrophic microorganisms has been estimated to be in the order of 1011 kg per year, about 60% of the earth’s newly fixed nitrogen.
- Lightning and ultraviolet radiation fix another 15%, with the remainder coming from industrial processes.
- Ammonia can also be obtained by the reduction of the nitrate ion (NO3–) that is present in the soil.
- Nitrate reduction can be carried out by most plants and microorganisms.
- The ammonia resulting from these two processes can then be assimilated by all organisms.
Reaction: N2 + 3 H2 –> <– 2 NH3
- This process is carried out by the nitrogenase complex, which consists of a reductase and an iron–molybdenum-containing nitrogenase.
- At least 16 ATP molecules are hydrolyzed to form two molecules of ammonia.
- Leghemoglobin is used to protect the nitrogenase in the Rhizobium from inactivation by O2.
2. Nitrification
- Nitrification is a two-step process in which NH3/ NH4+ is converted to NO3– .
- First, the soil bacteria Nitrosomonasand Nitrococcus convert NH3 to NO2–, and then another soil bacterium, Nitrobacter, oxidizes NO2– to NO3–.
- These bacteria are “chemotrophs” who obtain their energy from volatile chemicals. They gain energy through these conversions, both of which require oxygen to occur.
Nitrosomonas Nitrobacter
[NH4+] ————————— > NO2- ————————-> NO3-
Ammonium pH 4 – 10 nitrite pH 6 – 9 nitrate
Optimum conditions for nitrification:
- Adequate aeration
- Optimum temperature 25 – 35oC
- Adequate soil moisture
- Adequate exchangeable bases – particularly Calcium (Ca)
- NPK availability
- Requires a low C/N ratio
3. Nitrogen Assimilation
- The next step in the nitrogen cycle is the assimilation of inorganic nitrogen, into organic nitrogen-containing compounds.
- It is the process by which plants and animals incorporate the NO3– and ammonia formed through nitrogen fixation and nitrification.
- All organisms assimilate ammonia via two main reactions catalyzed by glutamate dehydrogenase and glutamine synthetase giving rise to the amino acids glutamate (Glu) and glutamine (Gln), respectively.
- The amino nitrogen in Glu and the amide nitrogen in Gln are then used in further biosynthetic reactions to give rise to other compounds.
Glutamate dehydrogenase
Glutamate dehydrogenase catalyzes the reductive amination of the citric acid cycle intermediate α-ketoglutarate. Although the reaction is reversible, the reductant used in the biosynthetic reaction is NADPH. This enzyme is also involved in the catabolism of amino acids.
Glutamine synthetase
Glutamine synthetase catalyzes the incorporation of ammonia into glutamine, deriving energy from the hydrolysis of ATP. This enzyme is named a synthetase, rather than a synthase because of the reaction couples bond formation with the hydrolysis of ATP. In contrast, a synthase does not require ATP.
- Plants take up these forms of nitrogen through their roots and incorporate them into plant proteins and nucleic acids. Animals are then able to utilize nitrogen from the plant tissues.
4. Ammonification
- Assimilation produces large quantities of organic nitrogen, including proteins, amino acids, and nucleic acids.
- Ammonification is the conversion of organic nitrogen into ammonia.
- Here, organic nitrogen from dead plants and soil organisms is converted to ammonium (NH4+) by mineralization. Mineralization is the decomposition of organic matter to NH4+ which is a useable nutrient ion.
- Many different soil organisms are involved in the ammonification process including bacteria and fungi. These soil organisms break down organic matter using the carbon and energy produced, but nitrogen is released.
- Ammonia is also released by the process of excretion into the environment and is then available for either nitrification or assimilation.
5. Denitrification
- Denitrification refers to the change (via denitrifying bacteria) of nitrate to gaseous forms of nitrogen such as nitrogen gas (N2) or nitrous oxide (N2O).
- The process is rapid and large amounts of N2 are lost from the soil system and end up in the atmosphere.
- This process only occurs where there is little to no oxygen, such as deep in the soil near the water table.
- Hence, areas such as wetlands provide a valuable place for reducing excess nitrogen levels via denitrification processes.
Conditions favoring denitrification:
- Lack of adequate O2
- Requires energy source of oxidizable organic matter for bacteria
- Warm, slightly acidic soils.
Significance of Nitrogen Cycle
The nitrogen cycle illustrates the relationship between different forms of nitrogen in the soil, water, air, and living organisms. It is considered to be a cycle since nitrogen moves around from place to place in different forms but is always present. It is important for the following reasons:
- Neither plants nor animals can obtain nitrogen directly from the atmosphere and thus depend on the nitrogen fixation process.
- Nitrogen (N) is an essential component of DNA, RNA, and proteins, the building blocks of life. All organisms require nitrogen to live and grow.
- Nitrogen is a key part of amino and nucleic acids and also an important part of ATP, which is the basic energy molecule for living things.
Nitrogen Cycle Key Terms
- NITROGEN FIXATION: The process of nitrogen gas is converted into ammonia by lightning or bacteria.
- NITRIFICATION: The process of ammonia being converted into nitrates and nitrites.
- AMMONIFICATION: The process of dead organisms breaking down into ammonia.
- DENITRIFICATION: The process of nitrates and nitrites being converted into nitrogen gas or gaseous nitrogen.
References
- David Hames and Nigel Hooper (2005). Biochemistry. Third ed. Taylor & Francis Group: New York.
- https://www.fondriest.com/news/nitrogencycle.htm
- https://www.acs.edu.au/info/sciences/chemical-sciences/what-is-the-nitrogen-cycle.aspx
- https://www.fondriest.com/news/nitrogencycle.htm
- https://biologydictionary.net/nitrogen-cycle/
- https://microbiologynotes.org/nitrogen-cycle/

Avery organise and agood notes,
thanks for the good information Sagar Aryal