Microbe Notes is an educational niche blog/website related to microbiology (bacteriology, virology, parasitology, mycology, immunology, molecular biology, biochemistry, etc.) useful for biology and microbiology courses (High School, B.Sc, M.Sc., M.Phil., and Ph.D.).
Choose Notes Categories
Latest Notes
- Red Blood Cells (RBCs): Structure, Life Cycle, Functions
- Scientific Method: Definition, Steps, Examples, Uses
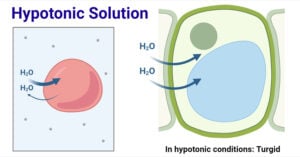
- Hypotonic Solution: Definition and Examples
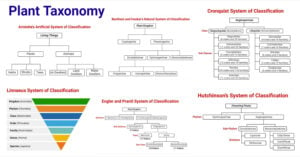
- Plant Taxonomy: Definition, Terms, Classifications
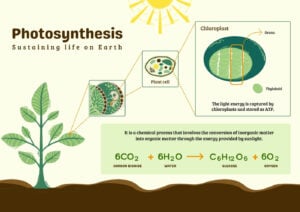
- Light Reaction and Dark Reaction of Photosynthesis
- Forest Biometrics: Definition, Tools, Methods, Uses
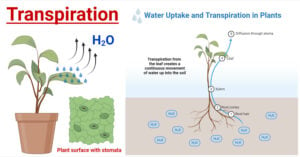
- Transpiration in Plants: Types, Mechanism, Factors, Uses
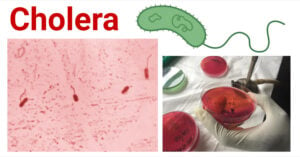
- Cholera: A-Level Biology Revision Notes
- Infectious Diseases: A-Level Biology Revision Notes
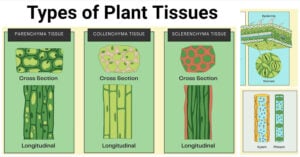
- Types of Plant Tissues: Meristematic and Permanent Tissue
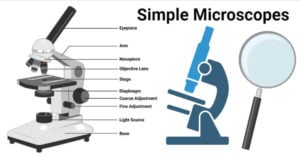
- Simple Microscope: Principle, Parts, Uses, Examples, Diagram
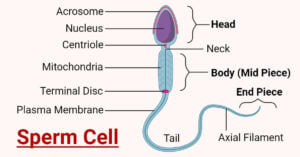
- Sperm Cell: Anatomy, Structure, Functions, Diseases
- Forest Economics and Its Implications
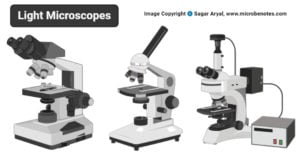
- Light Microscope: Principle, Types, Parts, Diagram
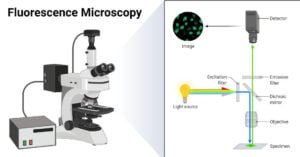
- Fluorescence Microscope: Principle, Parts, Uses, Examples
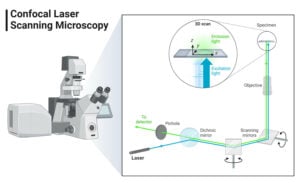
- Confocal Microscope: Principle, Parts, Types, Diagram, Uses
- Understanding Forest Ecology and Management
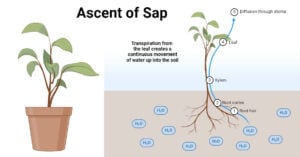
- Ascent of Sap: Definition, Mechanisms and Theories
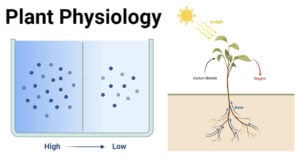
- Plant Physiology: Understanding the Life Processes of Plants
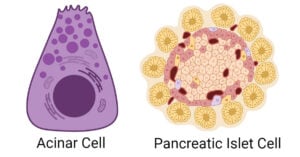
- Pancreatic Cells: Types, Structure, Functions, Diseases