Interesting Science Videos
What are Enzyme Inhibitors?
Inhibitors are compounds that convert the enzymes into inactive substances and thus adversely affect the rate of enzymatically-catalyzed reaction is called an enzyme inhibitor, and the process involved is termed enzyme inhibition. Some enzyme inhibitors are normal body metabolites that inhibit a particular enzyme while other inhibitors may be foreign substances, such as drugs or toxins. The inhibition may be a part of the normal cellular control of a metabolic pathway, a diseased condition, or either a therapeutic measure.
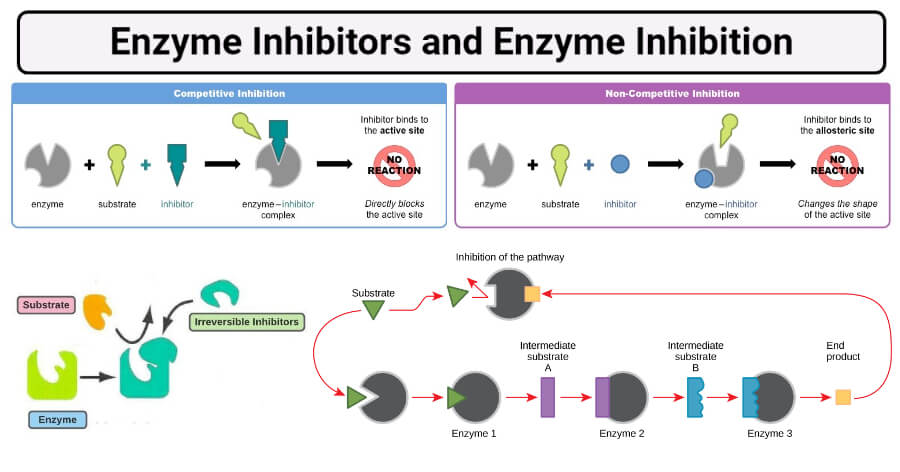
Image Source: BioNinja, Bio-Flix, OpenStax Biology.
Based on the mechanism of action of inhibitors, enzyme inhibition is classified into three groups:
1. Reversible Inhibition
Reversible inhibition is the inhibition of an enzyme caused by reversible inhibitors that dissociate very rapidly from its target enzyme because it becomes very loosely bound with the enzyme. Reversible inhibition is prevented by removing the inhibitor from the enzyme. Reversible inhibitors bind to enzymes with non-covalent interactions like hydrogen bonding and hydrophobic interactions. These weak interactions together result in strong binding. These inhibitors do not undergo chemical reactions but are easily removed by dilution or dialysis. Reversible inhibition are of three types; competitive inhibition, noncompetitive inhibition, and uncompetitive inhibition depending on three different factors:
- whether the inhibition can be overcome by increasing the concentration of the substrate,
- whether the inhibitor binds at the active site or allosteric site of the enzyme molecule
- whether the inhibitor binds either with the free enzyme only or with the enzyme-substrate complex only or with either of the two.
a. Competitive inhibition
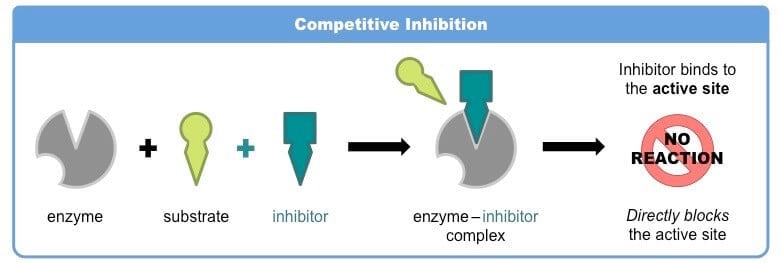
Image Source: BioNinja.
- Competitive inhibition is the inhibition of enzymatic activity by the competitive binding of inhibitors to the active site.
- The inhibitor is called a competitive inhibitor as it competes with the substrate to bind with the active site.
- The competitive inhibitor has a close structural resemblance with the substrate. The inhibitor may combine with the enzyme (E), forming an enzyme-inhibitor (EI) complex instead of an enzyme-substrate (ES) complex.
- The degree of inhibition depends upon the concentrations of the substrate and the inhibitor.
- Thus, inhibition can be increased by increasing the concentration of inhibitors and can be reduced by decreasing the concentration of the substrate.
- The action of a competitive inhibitor can be reversed at high substrate concentrations because a sufficiently high substrate concentration will successfully compete out the inhibitor molecule in binding to the active site.
- A competitive inhibitor diminishes the rate of the reaction by reducing the proportion of the enzyme molecules bound to a substrate.
- Example: Many microorganisms, like bacteria, synthesize the vitamin folic acid from para-aminobenzoic acid (PABA), and some sulfa drugs that are structural analogs of PABA act as an enzyme inhibitor and occupy the active site of some bacterial enzymes catalyzing this reaction.
b. Noncompetitive inhibition
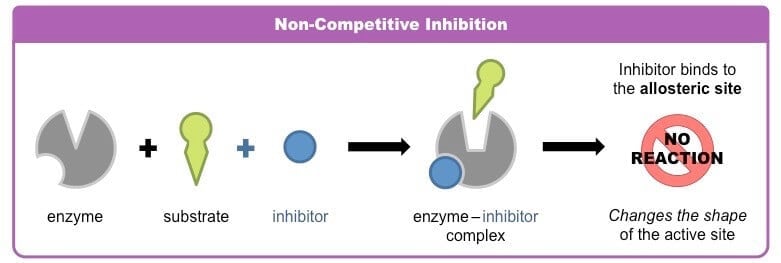
Image Source: BioNinja.
- Noncompetitive inhibition is the inhibition of enzymatic activity by the binding of inhibitors to the enzyme at a place other than the active site.
- The tern noncompetitive suggests that there is no competition between the substrate and the inhibitor for the binding to the active site and also has no structural resemblance to the substrate.
- The inhibitors involved in this process are termed noncompetitive inhibitors.
- Since inhibitor and substrate may combine at different sites, the formation of both enzyme-inhibitor complex and enzyme-substrate complexes takes place.
- In noncompetitive inhibition, the inhibitor and substrate can simultaneously bind to the same enzyme molecule since their binding sites are different and also, do not overlap.
- During competitive inhibition, the enzyme is inactivated when an inhibitor is bound, whether or not substrate is also present.
- The binding of the inhibitor brings about conformational changes in the active site of the enzyme, which prevents the binding of the substrate molecule.
- Besides, the inhibitor effectively lowers the concentration of active enzymes and hence lowers the rate of reaction.
- Noncompetitive inhibition, unlike competitive inhibition, cannot be overcome by increasing substrate concentration.
- Examples: Various heavy metal ions (Ag+, Hg2+, Pb2+) inhibit the activity of a variety of enzymes like urease.
c. Uncompetitive inhibition
- Uncompetitive inhibition is the inhibition of enzymatic activity by the binding of the inhibitor at an allosteric site like in the case of noncompetitive inhibition but the binding takes place with the enzyme-substrate (ES) complex, and not the free enzyme molecule.
- The mechanism of inhibition involved in uncompetitive inhibition is by the removal of an activated enzyme-substrate complex which causes a decrease in the maximum velocity of the chemical reaction.
- For example, tetramethylene sulfoxide and 3-butylthiolene oxide are uncompetitive inhibitors of liver alcohol dehydrogenase.
Read Also: Factors affecting enzyme action and immobilized enzymes
2. Irreversible inhibition
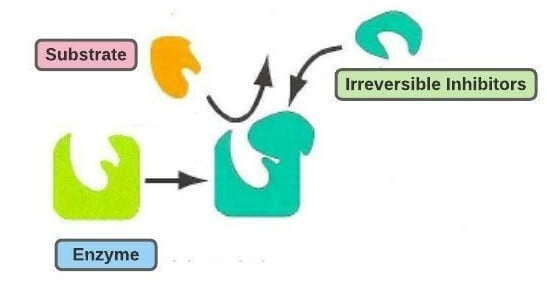
Image Source: Bio-Flix.
- Irreversible inhibition is the inhibition of enzymatic activity by the irreversible change in structure caused due to the binding of the inhibitor to the enzyme or by destroying some functional groups on the enzyme that is essential for its activity.
- An irreversible inhibitor separates very slowly from its target enzyme because it becomes very tightly bound to its active site while inactivating the enzyme molecule.
- Irreversible inhibition, unlike reversible inhibition, cannot be removed or reduced by the removal of inhibitors from the enzyme molecule.
- Irreversible inhibitors bind to the enzyme molecule by strong covalent bonds as they often contain reactive functional groups like aldehydes, alkene, and haloalkanes.
- Irreversible inhibitors are generally specific to one group of enzymes as they destroy the enzymes by altering the active site and not by destroying the structure of the proteins.
- The inhibition of enzymatic activity occurs in a time-dependent manner, usually occurring exponentially.
- Example: Alkylating reagents, like iodoacetamide act as irreversible inhibitors and inhibit the catalytic activity of some enzymes by modifying cysteine and other side chains.
3. End-product inhibition
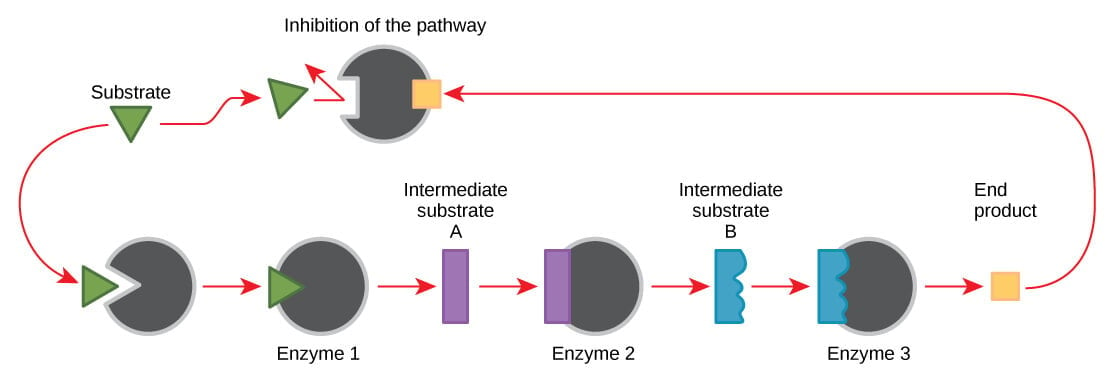
Figure: Metabolic pathways are a series of reactions catalyzed by multiple enzymes. Feedback inhibition, where the end product of the pathway inhibits an upstream step, is an important regulatory mechanism in cells. Image Source: OpenStax Biology.
- End-product inhibition is a cellular control mechanism in which the activity of enzymes is is inhibited by the enzyme’s end product.
- End-product inhibition is also termed feedback inhibition.
- This inhibition is involved in the regulation of how much of the end products to be produced.
- Most biochemical processes are complex and require multiple enzymes to produce the desired product. In such processes, end-product inhibition acts on the first enzyme of the process.
- End-product inhibitors bind to the allosteric site on the enzyme that causes changes in the shape of the enzyme, altering the active site.
- In feedback inhibition, the end product binds to the allosteric site, which causes changes in the enzyme.
- This inhibition is essential for living beings, as it helps the body avoid potentially dangerous conditions.
- For example: end-product inhibition is observed in humans where the enzyme responsible for the production of cholesterol by the liver is inhibited by the presence of cholesterol in the blood.
References and Sources
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Biologydictionary.net Editors. (2017, February 12). Feedback Inhibition. Retrieved from https://biologydictionary.net/feedback-inhibition/
- 5% – https://www.slideshare.net/pratham4012/enzyme-inhibitors-activation-energy-and
- 2% – https://www.slideshare.net/ErhardRutashobya/enzymes-63078197
- 2% – https://www.brainkart.com/article/Types-of-enzyme-inhibition_27766/
- 2% – https://www.bionity.com/en/encyclopedia/Enzyme_inhibitor.html
- 2% – https://quizlet.com/213807014/chapter-8-mechanisms-and-inhibitors-flash-cards/
- 2% – https://press.rebus.community/openstaxbiology/chapter/6-5-enzymes/
- 1% – https://www2.chem.wisc.edu/deptfiles/genchem/netorial/modules/biomolecules/modules/enzymes/enzyme6.htm
- 1% – https://www.wikidoc.org/index.php/Enzyme_inhibitor
- 1% – https://www.slideshare.net/fatimasaleh94214/enzymes-2-30256325
- 1% – https://www.freepatentsonline.com/y2004/0038923.html
- 1% – https://www.differencebetween.com/difference-between-competitive-and-vs-noncompetitive-inhibition/
- 1% – https://www.creative-enzymes.com/resource/Effect-Of-Enzyme-Inhibition-On-Enzymatic-Reaction_49.html
- 1% – https://www.chegg.com/homework-help/questions-and-answers/effect-competitive-inhibitor-reversed–answer–increasing-inhibitor-concentration-b-heatin-q3062830
- 1% – https://pediaa.com/what-is-the-difference-between-competitive-and-noncompetitive-inhibition/
- 1% – https://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Reversible_Inhibitors
- 1% – https://byjus.com/questions/what-is-the-allosteric-inhibitor-explain-with-an-example/
- 1% – http://dictionary.sensagent.com/Enzyme%20inhibitor/en-en/
- <1% – https://www.thefreedictionary.com/feedback+inhibition
- <1% – https://www.sciencedirect.com/topics/chemistry/enzyme-inhibitor
- <1% – https://www.sciencedirect.com/science/article/pii/B9780080500140500110
- <1% – https://www.dsm.com/markets/anh/en_US/Compendium/companion_animals/folic_acid.html
- <1% – https://www.britannica.com/science/metabolism/End-product-inhibition
- <1% – https://quizlet.com/40464732/chem-exam-4-flash-cards/
- <1% – https://pubs.acs.org/doi/10.1021/cb400411x
- <1% – https://ocw.mit.edu/courses/chemical-engineering/10-492-2-integrated-chemical-engineering-topics-i-introduction-to-biocatalysis-fall-2004/lecture-notes/lecture4.pdf
- <1% – https://en.m.wikipedia.org/wiki/Product_inhibition
- <1% – https://bio.libretexts.org/Bookshelves/Biochemistry/Book%3A_Biochemistry_Free_For_All_(Ahern_Rajagopal_and_Tan)/04%3A_Catalysis/4.02%3A_Control_of_Enzymatic_Activity
