Interesting Science Videos
What is 3D Bioprinting?
3D Bioprinting is the method of printing biomedical structures with the use of viable cells, biological molecules, and biomaterials.
- In simple words, 3D bioprinting is the deposition of biological material in a layer-by-layer fashion to create 3D structures like tissues and organs.
- Bioprinting is considered a part of additive manufacturing that involves the formation of materials necessary in industrial applications.
- 3D bioprinting begins with a suitable microarchitecture which is further stabilized by scaffolds of cells and tissues while considering the effect of manufacturing on cell viability.
- The most important motivation behind the development of 3D bioprinting is the limited availability of biological structures that are required for the rehabilitation of lost organs and tissues.
- The ultimate aim of the process is to provide an appropriate alternative to tissue implants and animal testing procedures during research on diseases and the development of treatments.
- Currently, the use of 3D bioprinting is limited to the formation of organs and tissues to estimate the efficiency of drugs, but 3D bioprinting has great scope in its use for replacing lost and failed organs in patients.
- 3D bioprinting is trickier than 3D printing as the cells are more sensitive and require special attention to allow the cells to grow and divide and prevent the cytotoxic activity of solvents used during the process.
- The research on 3D bioprinting is focused on the development of approaches that allow the fabrication of 3D functional living structures of biological and mechanical importance in order to restore the functions of tissues and organs.
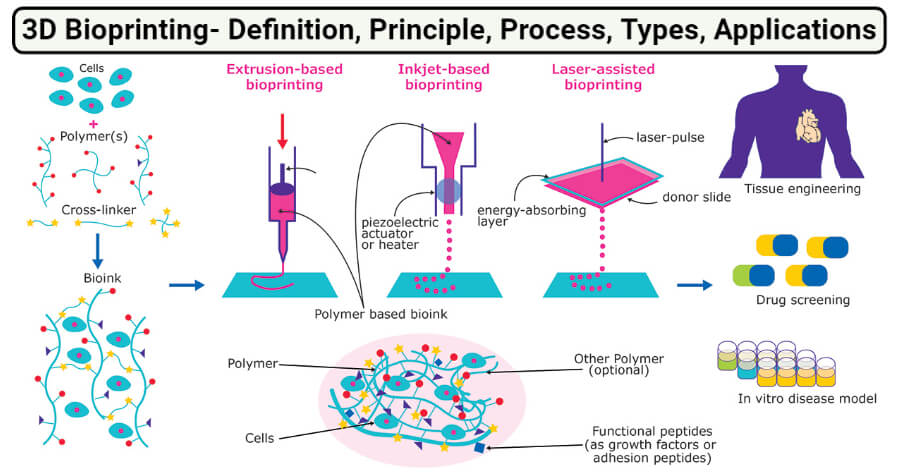
Image Source: Merck.
What are Bioinks?
Bioinks are biological materials used in the manufacture of engineered live tissues by the process of 3D bioprinting.
- The term bioink doesn’t only indicate the cells used in manufacturing, but also carrier molecules that provide support to the growing cells.
- Common carrier materials used with cells during bioprinting are biopolymer gels that act as a 3D molecular scaffold so that cells can attach, grow, and increase.
- The biopolymers used in bioink are essential as they retain water which provides mechanical stability to the engineered tissues.
- The selection of bioink for a particular process is an important step as the selected bioinks should have desired physicochemical properties that include mechanical, chemical, biological, and rheological characteristics.
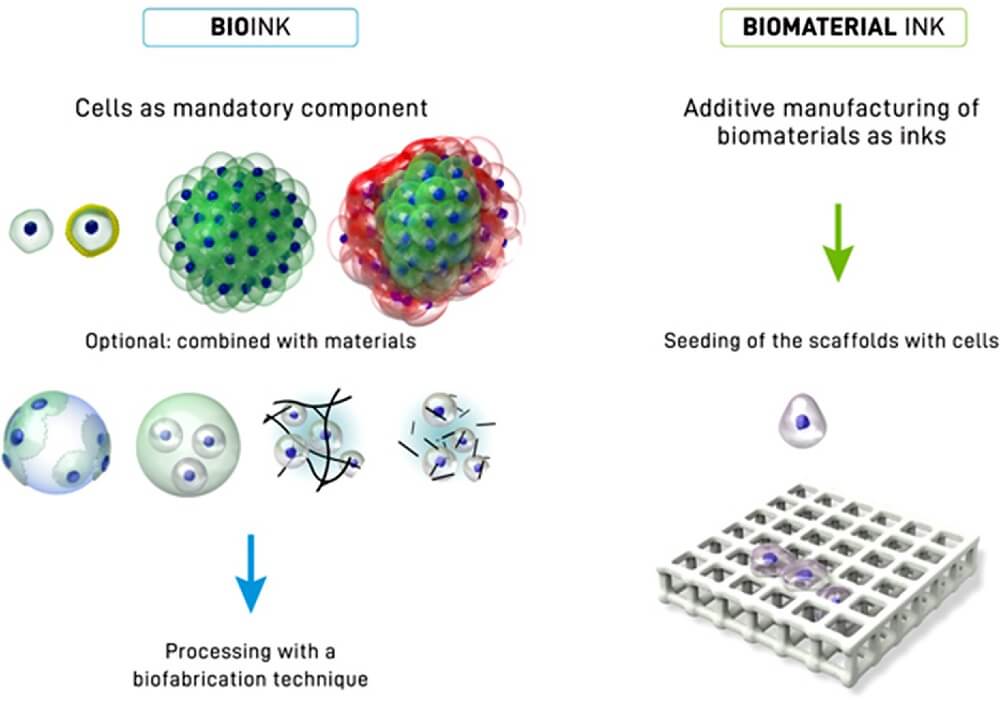
Figure: Distinction between a bioink (left side) and a biomaterial ink (right side). In a bioink, cells are a mandatory component of the printing formulation in the form of single cells, coated cells or cell aggregates (of one or several cell types), or also in combination with materials (for example seeded onto microcarriers, embedded in microgels, formulated in a physical hydrogel, or formulated with hydrogel precursors). In the case of biomaterial ink, in principle any biomaterial can be used for printing and cell-seeding occurs post-fabrication. Image Source: https://doi.org/10.1016/j.actbio.2020.06.040.
The bioinks used in the bioprinting process should have the following properties:
- The bioinks used should be able to provide adequate mechanical strength and robustness while maintaining the tissue-matching mechanics in the resulting tissue constructs.
- The bioink molecules should have adjustable gelation and stabilization to result in high shape fidelity during bioprinting.
- The bioinks should be biocompatible and can undergo biodegradability according to the natural microenvironment of the tissue.
- The bioinks should be suitable for chemical modifications to form specific tissues.
Basic Principle of 3D Bioprinting
The principle of 3D printing is based on the precise placement of biological components, biochemicals, and living cells in a layer-by-layer fashion with the spatial control of the placement of functional constituents onto the fabricated 3D structure. The process of 3D bioprinting is based on three distinct approaches; biomimicry or biomimetics, autonomous self-assembly, and mini-tissue building blocks.
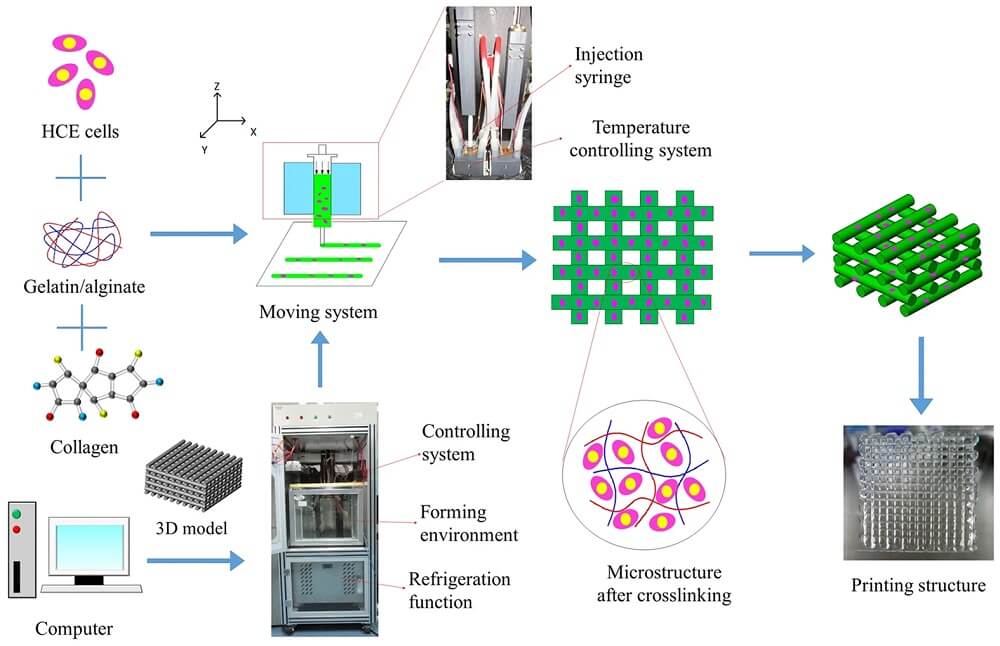
Figure: Schematic illustration of the 3D bioprinting process and optical images of the printing set up and printing constructs. Image Source: Springer Nature.
1. Biomimicry
- Biomimicry is the manufacture of identical reproductions of cellular and extracellular components of tissues and organs after a detailed examination of nature itself.
- In order to achieve biomimicry, specific cellular functional components of tissues are to be precisely reproduced.
- Since the materials used in the process have a significant influence on cell attachment, cell size, and morphology, the control of proliferation and differentiation of cells is present in the scaffold.
- A detailed understanding of the microenvironment, including the arrangement of cell types, composition of the extracellular matrix, a gradient of soluble and insoluble factors, and the nature of biological forces is required.
2. Autonomous self-assembly
- Autonomous self-assembly is the approach of replicating biological tissue by using the mechanism of embryonic tissue and organ development as a guide.
- The cellular component of a developing tissue produces its own extracellular matrix and cell signals that allow autonomous organization and patterning to form the desired microarchitecture.
- During the process, a scaffold-free version is formed using self-assembling cellular spheroids that undergo differentiation and organization to form the desired tissue.
- This approach relies on the cell as the primary driver of tissue formation, which directs the localization, functioning, and structure of the resulting tissue.
- The use of this approach requires detailed knowledge of the developmental mechanism of embryonic tissues and organogenesis.
3. Mini tissues building blocks
- Mini tissue building blocks approach utilizes the method of both of the previous strategies.
- In this method of bioprinting, small functional units of tissues and organs, called mini-tissues, are formed.
- The mini tissues represent the smallest structural and functional unit of the organs, like the kidney neuron.
- These mini-tissues can then be fabricated either via autonomous self-assembly or biomimicry.
- The bioprinting begins with the assembly of mini-tissues into macro-tissues based on biologically inspired organization, which is then followed by the reproduction of tissue units that can self-assemble to form functional structures.
Basic Steps of 3D Bioprinting (process)
The overall process of 3D bioprinting can be achieved via three distinct steps; pre-bioprinting, bioprinting, and post-bioprinting.
1. Prebioprinting
- The first step of prebioprinting is the formation of a model that is used by the printer and the choosing of materials to be used during the process.
- It begins with the extraction of biopsy of a tissue which provides a biological model that is to be recreated by the 3D bioprinting method.
- Technologies like computed tomography (CT) or magnetic resonance imaging (MRI) scans are used in this step.
- The images obtained through these methods are tomographically reconstructed to obtain 2D images.
- Cells necessary for the process are then selected and multiplied. The cell mass thus formed is mixed with oxygen and other nutrients to keep them viable.
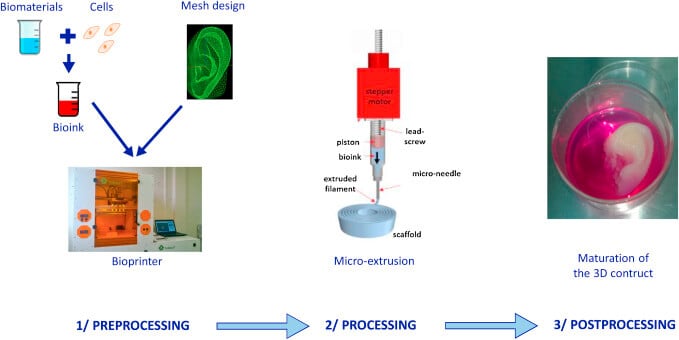
Image Source: https://doi.org/10.1016/j.jormas.2018.12.014.
2. Bioprinting
- The second step is the actual printing process where the bioink is placed in the printer to form a 3D structure.
- The mixture of cells, nutrients, and matrix, together forming bioink, is then placed onto the printer cartridge, which then deposits the material based on the digital model prepared.
- The formation of biological constructs involves the deposition of bioink onto the scaffold in a layer-by-layer approach to generate a 3D tissue structure.
- This step of the bioprinting process is a complex process as it requires the formation of different cell types based on the type of tissues and organs to be formed.
3. Postbioprinting
- Postbioprinting is the last step of the bioprinting process, which is important to provide stability to the printed structure.
- In order to maintain the structure and function of the biological matter, physical and chemical stimulations are required.
- These stimulations provide signals to the cells to reorganize and maintain the growth of tissues.
- In the absence of this step, the mechanical structure of the material might be disrupted, which then affects the functioning of the material.
3D Bioprinting Technology (Types)
1. Extrusion based bioprinting
- Extrusion-based bioprinting or microextrusion is the most common method of printing non-biological 3D structures.
- This bioprinting technology is used in various academic institutions for tissue and organ research.
- The flexibility of the process and material availability makes extrusion-based 3D bioprinting, the most used technique to produce pharmaceutical dosage forms.
- The printers used in this method have a temperature-controlled material handling and dispensing system with a stage, both of which are capable of moving along the x, y, and z axes.
- Besides, the system also consists of a fiberoptic light source to illuminate the deposition area for photoinitiator activation (if required).
- Some of the microextrusion bioprinters utilize multiple print heads to allow serial dispensation of several materials at once.
Principle of Extrusion based bioprinting
- The extrusion-based 3D bioprinting utilizes one of the two mechanisms to produce the desired result; the semi-solid extrusion (SSE) and the fused deposition modeling (FDM) based 3D printing.
- In the SSE based 3D bioprinting, pressurized air or rotating screw gear is used to extrude a continuous stream of semi-solid materials through a nozzle which is deposited in a layer-by-layer fashion to form a 3D structure.
- The FDM 3D bioprinting, however, utilizes high temperature to melt thermoplastic filaments which are then extruded through a nozzle to deposit in a layer-by-layer fashion to produce a 3D structure.
- The two principal components of all extrusion-based 3D printers include the extrusion system and the positioning system; thus, both of these systems should be accurate enough to produce a visually and geometrically accurate structure.
Examples of Extrusion based bioprinting
- The extrusion-based 3D bioprinting method has been widely used in various biomedical sectors ranging from the pharmaceutical industry to research sectors.
- The technology is commonly used for single tissue applications, and to manufacture scaffolds that mimic tissue interfaces.
- The technology is capable of producing models that mimic soft tissues and bone structures which provide an opportunity for possible implants.
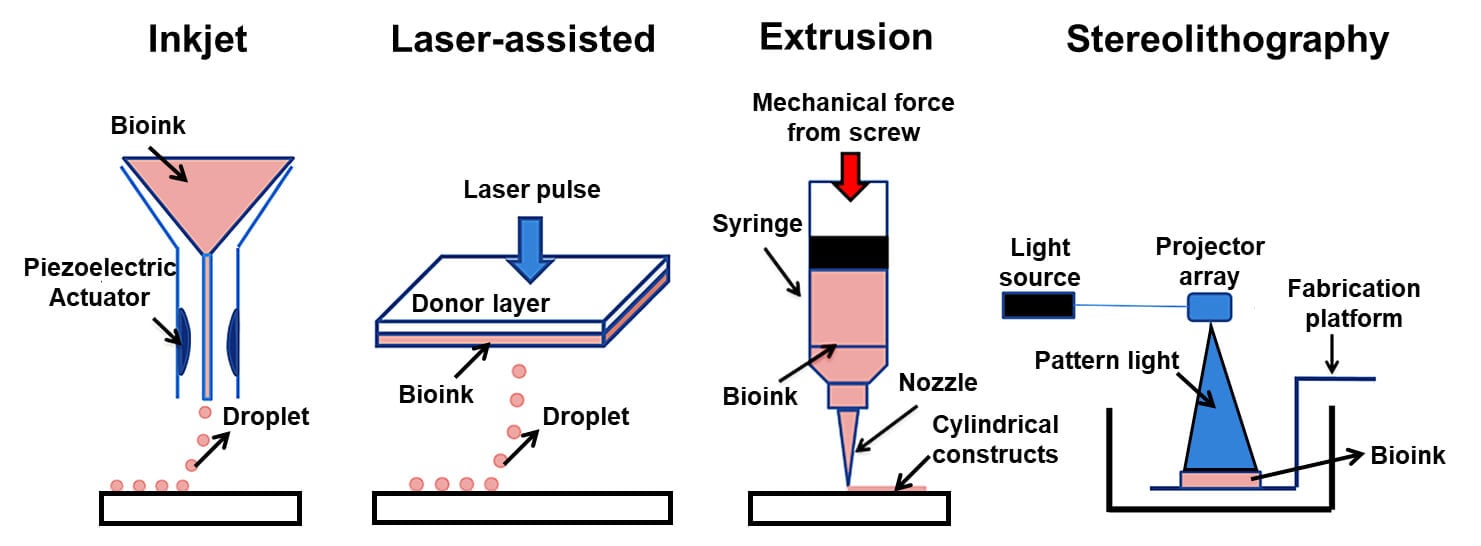
Image Source: https://doi.org/10.20900/rmf20190004.
2. Inkjet-based bioprinting
- Inkjet bioprinting or drop-on-demand bioprinting is the most commonly used technology for both non-biological and biological applications.
- This technology was initially only used for 2D ink-based printing but was later modified by replacing the ink in the cartridge with biological material, and the paper was replaced by an electronically controlled elevator stage to provide control.
- Currently, inkjet bioprinting can be performed on bioprinters that are custom-designed to handle and print biological materials with high precision, speed, and resolution.
- One of the limitations of inkjet bioprinting is that the biological materials have to be in a liquid form to enable droplet formation.
Principle of Inkjet-based bioprinting
- Inkjet bioprinting is based on the ejection of drops of liquid onto a substrate by thermal or acoustic forces.
- Thermal inkjet bioprinting can be achieved by electrically heating the print head to generate pressure that causes the release of droplets from the nozzle.
- In the case of acoustic inkjet bioprinting, a piezoelectric crystal is used that creates an acoustic wave inside the print head to break the liquid into droplets.
- When a voltage is applied to a piezoelectric substance, a rapid change in shape is induced. This, in turn, generates pressure required to force droplets out of the nozzle.
- Both of these methods have their own advantages and disadvantages; thus, the selection of inkjet bioprinting technology should be made based on the desired purpose.
Examples of Inkjet-based bioprinting
- Some of the common applications of inkjet bioprinting are the regeneration of functional skin and cartilage tissues where the high printing speed of this technique enables direct deposition of cells and biomaterials onto skin and cartilage lesions.
- Besides, inkjet bioprinting also allows the deposition of primary or stem cells with uniform density onto lesions while maintaining cell viability and function.
- Layered cartilage constructs have also been developed using a combination of inkjet bioprinting and electrospinning technology.
3. Pressure-assisted bioprinting (PAB)
- Pressure-assisted bioprinting is based on the extrusion of biomaterials out of the nozzle of the printer in order to fabricate a 3D biological structure.
- Some of the common biomaterial used in this method include hydrogels, cells and proteins, and ceramic material solutions, collagen and chitosan, etc.
- The speed of the printers remains low, and it provides about 40-80% cell viability.
- The use of pressure-assisted bioprinting allows room temperature processing and direct incorporation of homogenous cells onto the substrate.
Principle of Pressure-assisted bioprinting (PAB)
- The pressure is created by a coordinated motion of pneumatic pressure or plunger or via screw-based pressure in the form of the continuous filament.
- The deposition of materials occurs on a stationary substrate in a layer-by-layer fashion to obtain a complete 3D structure.
Examples of Pressure-assisted bioprinting (PAB)
- Pressure-based bioprinting has been used in the printing of cells and organs with functional activity.
- The technique has been used to produce human mesenchymal cells, endothelial cells, and osteogenic progenitors.
- Besides, the method can also be used to obtain multicellular bioprinted constructs with retention of heterogeneous cell organization in various mammalian bodies.
- The mesenchymal cell constructs obtained via pressure-based bioprinting can then be differentiated into other cells that can retain activity in vivo.
4. Laser-assisted bioprinting (LAB)
- Laser-assisted bioprinting is the method of depositing biomaterials onto a surface by using a laser as a source of energy.
- Traditionally, this technique was limited to transferring metals, but it has since been modified to be applied to biological materials like cells, DNA, and peptides.
- A laser-assisted bioprinter consists of a pulsed laser beam, a focusing system, a ribbon with donor transport support, a layer of biological material prepared in a liquid solution with a receiving substrate facing the projector.
- The biomaterials to be used in laser-assisted bioprinting include a hydrogel, culture media, cells, proteins, and ceramic materials.
- The speed of the bioprinters is medium, and the method retains about 95% of cell viability.
Principle of Laser-assisted bioprinting (LAB)
- The principle of laser-assisted bioprinting is the use of the laser to induce forward transfer of biomaterials onto a solid surface.
- The laser present on the printer irradiates the ribbon, which causes the liquid biomaterial to evaporate and reach the receiving substrate in droplet form.
- The receiving substrate consists of biopolymers or a cell culture medium which assists cellular adhesion and sustained growth of the biomaterial.
Examples of Laser-assisted bioprinting (LAB)
- Laser-assisted bioprinting has been used to produce a cellularized skin constructs with relevant cell densities in a layered tissue construct.
- Cells of the human dermal fibroblasts, pulmonary artery endothelial cells, and breast cancer cells can be produced via laser-assisted bioprinting.
5. Stereolithography (STL)
- Stereolithography is a freeform, nozzle free technique used to produce the 3D structure of biological and non-biological materials.
- The stereolithography technique has the highest fabrication accuracy, and a large number of materials can be used in the process.
- The technique utilizes light-sensitive hydrogels that are deposited in a layer-by-layer fashion to form a 3D structure.
- The speed of this method is very fast (about 40,000 mm/s) with cell viability of more than 90%.
Principle of Stereolithography (STL)
- Stereolithography technology is based on the solidification of the liquid photosensitive polymer upon illumination.
- The technology utilizes digital micromirror arrays to control the light intensity to polymerize light-sensitive polymer materials.
- The photochemical solidification of biopolymers results in the formation of layers that together form a 3D object.
Examples Stereolithography (STL)
- This technique has been used in several ways to produce tissues and organs of different animals, including humans.
- Besides, the technique was tested upon on DNA material, but the use of UV light has chances of affecting the DNA structure. However, a custom light source can be prepared to use with DNA molecules.
What are Bioprinters?
Bioprinters or 3D bioprinters are automated devices for the additive fabrication of 3D functional tissues and organs based on the digital models that are created via various scans using biomaterials.
- Bioprinters are automated robotic devices that work on the basis of different mechanisms.
- 3D printers that can only print cell-free scaffolds but cannot dispense living cells are not considered bioprinters.
- The first commercial 3D bioprinter was prepared in Germany at Freiburg University by Prof. Ralf Mulhaupt’s group.
- The evolution of 3D bioprinters is a continuous process that involved the hybridization of new technological approaches to creating new advanced forms of bioprinters.
- There are different types of bioprinters depending on the technique of bioprinting employed by the machines; inkjet bioprinters, extrusion-based bioprinters, and laser-based bioprinters.
- These bioprinters work on different mechanisms and are generally used for different purposes depending on the type of biomaterials used.
Bioprinter Components
- The size of the printers is dictated by the functional specifications depending on the desirable bioprinted tissue or organ construct.
- The number of nozzles or openings also depends on the functional specification of the device.
- Other specific components, like laser sources and temperature controls, are different in different types of bioprinters.
- Different types of bioprinters have different components, but these bioprinters share some common characteristics with five main structural-functional components; robotic positioning in the X-Y-Z axis, nozzle or disperser or extrusion machine, operational or controlling system, and receiver substrate.
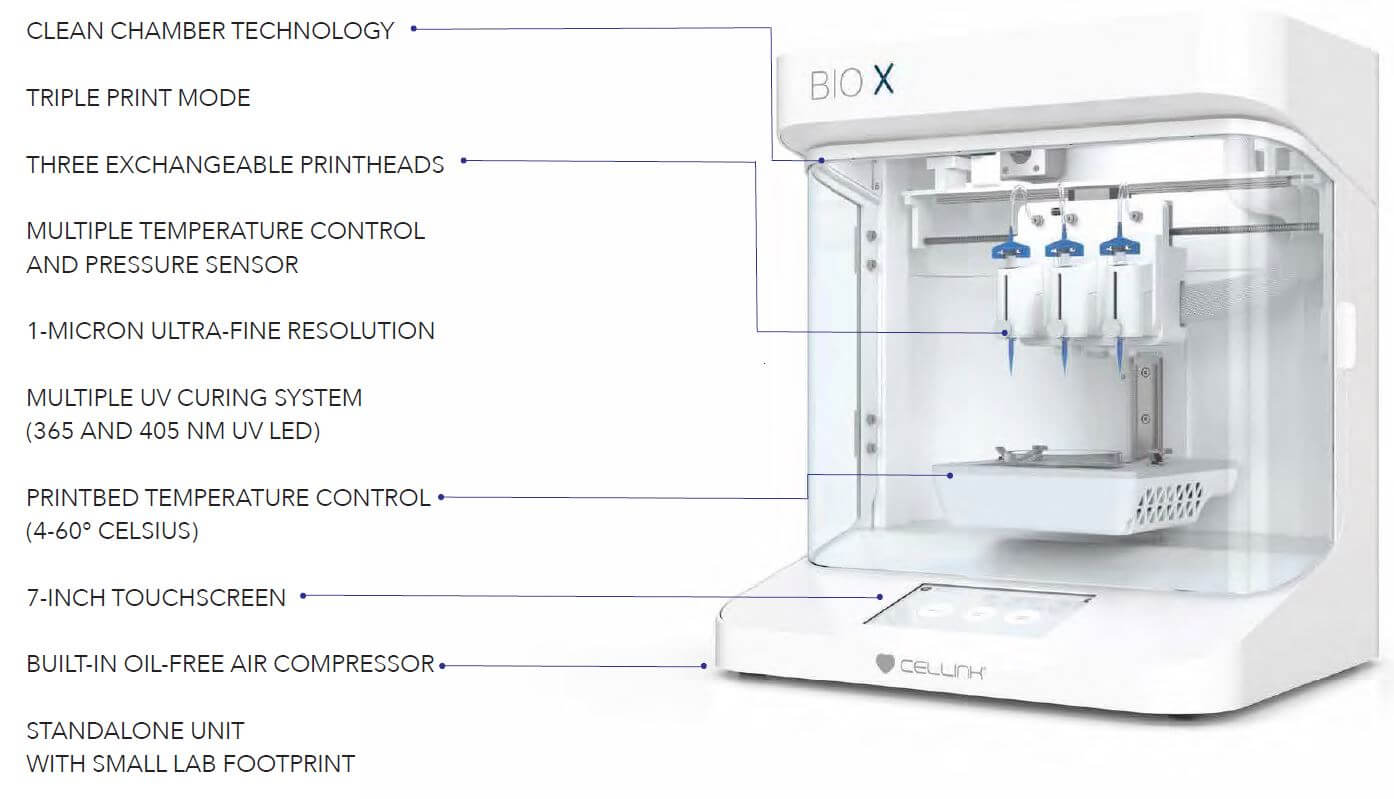
Image Source: Cellink.
Anatomically, the following are different parts of bioprinters;
a. Head mount
- The head of the printer is attached to a metal plate that runs along the horizontal axis. The motor on the x-axis moves the metal plate side to side to deposit the biomaterial in a horizontal direction.
b. Elevator
- The elevator is the metal track running vertically at the back of the machine. It is driven by the z-axis motor that moves the head of the printer in an up and down direction.
c. Platform
- The platform is a shelf present at the bottom of the machine that provides a space for the organ to rest during the fabrication process.
- The platform can either be a scaffold or a Petri dish. A third motor is also present in the printer that moves the platform along the y-axis.
d. Reservoirs
- The reservoir is present on the print head that holds the biomaterial that is to be deposited during the printing process.
e. Nozzle
- The biomaterial in the reservoir present in the print head is forced out through a small nozzle or syringe which is present just above the platform.
How do Bioprinter works?
- The process of bioprinting begins with the CT or MRI scans of the desired organ. The image thus obtained is loaded into a computer that builds a corresponding 3D blueprint of the organ using a software program.
- The information from the 3D data is combined with histological information based on microscopic analysis to produce a layer-by-layer model of the organ.
- The information is then fed into the printer. Besides, other information about the choice of material to be used is also entered into the printer.
- The printer then reads the blueprint and deposits the biomaterial onto the receiver in a layer-by-fashion.
- It is achieved by the movement of the print head in all directions to generate the required depth and thickness.
- Once a layer reaches the platform, it solidifies either by cooling or via a chemical reaction. To the solidified layer, a new layer is deposited to form a stable structure.
- The organ thus formed is removed from the printer and placed in an incubator to allow the structures to settle and stabilize.
Examples of 3D bioprinters
- 3D bioprinters are developed by different companies like Cellink that can create cartilages, skin, bones, and muscles.
- Depending on the mechanism of bioprinting utilized, bioprinters like inkjet printers, laser printers, etc. are used for different purposes.
Applications of 3D Bioprinting
1. Tissue engineering
- Tissue engineering is one of the most prominent applications of 3D bioprinting. It enables the fabrication of complex tissues and organs that can replace failed or lost tissues.
- Production of functional tissues and organs at clinically relevant dimensions is challenging as the integration of the vascular network of arteries and veins and incorporation of various cell types to reinvent complex organ biology are not easy to achieve.
- Nevertheless, a wide variety of tissues have been successfully bioprinted while maintaining mechanical integrity and functioning.
Some of the common examples of tissues that have been bioprinted for various purposes:
a. Skin
- Skin tissue fabrication is achieved by a number of tissue engineering approaches.
- Tissue engineering can be done to produce substitutes like an autologous split-thickness skin graft, allografts, acellular dermal substitutes, and cellularized graft-like commercial products.
- Bioprinting of skin tissue can be done using an eight-channel valve-based bioprinter where a 13-layer tissue is constructed using collagen hydrogel.
- Keratinocytes are then bioprinted on top of alternating layers of human foreskin fibroblasts and acellular collagen layers to fabricate constructs with densely packed cells in epidermal layers.
- The tissue constructs prepared are engrafted with the host after about 10 days in the stratified epidermis.
- This results in early signs of differentiation and formation of the stratum corneum as well as some blood vessels.
- The biomaterial used for the process might differ but the most common cells used are keratinocytes and fibroblasts.
- Besides, skin with infections or diseases can be used as biomaterials for bioprinting to study the pathophysiology of the disease.
b. Bone and cartilage
- Bone and cartilage fabrication is the most mature use of bioprinting as the composition of such hard tissues is uncomplicated and is mostly composed of inorganic elements.
- Even though other techniques like gas foaming, salt leaching, and freeze-drying have been employed to produce such hard tissues, 3D bioprinting produces the most accurate structures.
- Thermal inkjet bioprinter is used to fabricate polymethacrylate scaffolds from bone-marrow-derived human mesenchymal stem cells.
- The cells are coprinted with nanoparticles of bioactive glass to control the spatial placement of cells.
- In cartilage tissue engineering, a printable bioink is prepared as a combination of nano fibrillated cellulose and alginate with human chondrocytes as living soft tissue.
c. Blood vessels
- Bioprinting of vascular networks is essential as the fabrication of tissues and organs depends on vascularization to provide oxygen and media to the bioprinted constructs.
- The bioprinting technology used for the production of bioprinted vascular networks includes extrusion- and laser-assisted bioprinting technique.
- During bioprinting, hydrogen gels including sodium alginates and chitosan are bioprinted directly in tubular form with encapsulated cells.
- The tubular structures thus formed have improved metabolic transportation and cellular viability.
d. Liver tissue
- Bioprinting of liver tissue is comparatively less prevalent as the cells of the liver have strong regeneration ability.
- However, there is a limitation of healthy donors, and the regeneration period for such liver is long.
- The bioink used for this purpose include cells like primary hepatocytes and stem-cell-derived hepatocytes.
- 3D printing technology can provide the exact size and shape of the liver, which is suitable for fo the patient.
- Bioprinting produces canaliculi that are linked together by the collagen matrix to form larger structures.
2. Drug development/screening
- Drug discovery requires time-consuming and costly processes that demand substantial financial investment and workforce.
- Thus, the development of a technique to improve the ability to predict the efficacy and toxicity of newly developed drugs earlier in the drug discovery process helps in reducing the time and money required.
- Bioprinting can fabricate 3D tissue models that resemble that of native tissue and are capable of high throughput assays.
- Most commonly, liver and tumor tissues are the primary focus to create tissue models for pharmaceuticals.
- Besides, depending on the target cells of developed drugs, the tissue models of such cells can be prepared and tested.
- Initially, tissue constructs of epithelial cells are prepared as these cells form the lining through which the drug diffuses into the bloodstream.
- Based on the studies on such constructs, the path of drugs and their action on the target cells can be assumed.
- Similarly, bioprinting can be used as an alternate way for the development of prescription drugs.
- The drugs can even be customized for each patient by preparing appropriate doses of drug print by using a set of biochemical inks.
- 3D printed composite pills containing multiple drugs with unique release rates can be used instead of taking multiple pills throughout the day.
3. Toxicology Screening
- Toxicology screening or testing is the process of identifying potential adverse effects of chemicals on individuals or to the environment.
- Chemicals might include compounds like pharmaceutical ingredients, cosmetic ingredients, household, and industrial chemicals.
- Studies evaluating the toxicity of some chemicals might require a larger number of human subjects with diverse metabolism, which might seem unethical.
- Some studies can be performed on animals, but animal might not predict human responses to an accurate or reliable manner.
- Instead, the use of 3D bioprinting can provide a highly-automated and advanced technology that can produce constructs that mimic the structure and function of human tissues.
- The use of such constructs facilitates real-time monitoring and high throughput screening of various chemicals.
- Testing of cosmetic ingredients on human-relevant skin tissue models has been performed for a long time.
- These tests study skin absorption, skin irritation, skin corrosion, and skin sensitization on models mimicking human tissue structures.
4. Tissue model for cancer research
- 2D tumor models have been used in cancer research for a long time, but those do not represent the physiologically relevant environment as the 2D models lack cell-cell interactions.
- However, 3D bioprinting allows the recapitulation of the cancer microenvironment so as to study cancer pathogenesis and metastasis accurately.
- Multiple cell types can be simultaneously bioprinted to form multicellular structures in a reproducible manner with a spatially mediated microenvironment and controlled cell density and cell-cell distance.
- Bioprinting of HeLa cells can be done in a gelatin-alginate composite hydrogel so as to study cell aggregation.
- These tissues can be used to study the progression of cancer and the changes in tissue structure and function with the progression.
- Besides, tissue models can also be used to study the efficiency of treatment methods against various carcinogens.
Limitations and Future Challenges of 3D Bioprinting
- The primary barriers in bioprinting are suitable bioinks with high biocompatibility and mechanical strength.
- Bioprinter technology that is currently used has comparatively lower resolution and speed, which produces a challenge for future development. Similarly, the bioprinters should also be compatible with a wide spectrum of biomaterials.
- The speed of the bioprinting process should be increased to mass-produced biomaterials at a commercially acceptable level as the current speed is slow.
- Vasculature of tissue constructs is an important challenge in 3D bioprinting as the tissues require continuous oxygen and nutrients.
- There are some ethical issues with 3D bioprinting as the cost of the method might make it inaccessible to the poor.
- Because bioprinting is a novel technology, it should be studied sufficiently to ensure it is going to be safe for humans.
- Personalized 3D printing technology might lead to a serried of regulatory problems to ensure printed product supervision.
References
- Papaioannou, Theodore G et al. “3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication.” Acta Cardiologica Sinica vol. 35,3 (2019): 284-289. doi:10.6515/ACS.201905_35(3).20181115A
- Murphy, S., Atala, A. 3D bioprinting of tissues and organs. Nat Biotechnol 32, 773–785 (2014). https://doi.org/10.1038/nbt.2958
- Kačarević, Željka P et al. “An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects.” Materials (Basel, Switzerland) vol. 11,11 2199. 6 Nov. 2018, doi:10.3390/ma11112199
- Dey, Madhuri, and Ibrahim T Ozbolat. “3D bioprinting of cells, tissues and organs.” Scientific reports vol. 10,1 14023. 18 Aug. 2020, doi:10.1038/s41598-020-70086-y
- Hsieh FY, Hsu SH. 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis. 2015;11(4):153-8. doi: 10.1080/15476278.2015.1123360. PMID: 26709633; PMCID: PMC4879895.
- N Sigaux, L Pourchet, P Breton, S Brosset, A Louvrier, CA Marquette. 3D Bioprinting:principles, fantasies and prospects. Journal of Stomatology, Oral and Maxillofacial Surgery. Volume 120. 2019. Pages 128-132. https://doi.org/10.1016/j.jormas.2018.12.014.
- Placone, Jesse K, and Adam J Engler. “Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications.” Advanced healthcare materials vol. 7,8 (2018): e1701161. doi:10.1002/adhm.201701161
- Algahtani, Mohammed & Mohammed, Abdul & Ahmad, Javed. (2019). Extrusion-Based 3D Printing for Pharmaceuticals: Contemporary Research and Applications. Current Pharmaceutical Design. 25. 10.2174/1381612825666190110155931.
- Li, Jipeng et al. “Recent advances in bioprinting techniques: approaches, applications and future prospects.” Journal of translational medicine vol. 14 271. 20 Sep. 2016, doi:10.1186/s12967-016-1028-0
- Pereira F.D.A.S., Parfenov V., Khesuani Y.D., Ovsianikov A., Mironov V. (2018) Commercial 3D Bioprinters. In: Ovsianikov A., Yoo J., Mironov V. (eds) 3D Printing and Biofabrication. Reference Series in Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-45444-3_12
- Ozbolat, Ibrahim & Peng, Weijie & Ozbolat, Veli. (2016). Application areas of 3D bioprinting. Drug Discovery Today. 21. 10.1016/j.drudis.2016.04.006.
- Saygili, E., Dogan-Gurbuz, A. A., Yesil-Celiktas, O., & Draz, M. S. (2019). 3D Bioprinting: A Powerful Tool to Leverage Tissue Engineering and Microbial Systems. Bioprinting, e00071. doi:10.1016/j.bprint.2019.e00071
- Nie, J., Gao, Q., Fu, J., He, Y., Grafting of 3D Bioprinting to In Vitro Drug Screening: A Review. Adv. Healthcare Mater. 2020, 9, 1901773. https://doi.org/10.1002/adhm.201901773
- Ng, Wei Long & Yeong, Wai Yee. (2019). The Future of Skin Toxicology Testing – 3D Bioprinting Meets Microfluidics. 5. 237. 10.18063/ijb.v5i2.1.237.
- Peng, Weijie & Datta, Pallab & Ayan, Bugra & Ozbolat, Veli & Sosnoski, Donna & Ozbolat, Ibrahim. (2017). 3D Bioprinting for Drug Discovery and Development in Pharmaceutics. Acta Biomaterialia. 57. 10.1016/j.actbio.2017.05.025.
- Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, Chen S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv Drug Deliv Rev. 2018 Jul;132:235-251. doi: 10.1016/j.addr.2018.06.011. Epub 2018 Jun 21. PMID: 29935988; PMCID: PMC6226327.
- Ecem Saygili, Asli Aybike Dogan-Gurbuz, Ozlem Yesil-Celiktas, Mohamed S. Draz. 3D bioprinting: A powerful tool to leverage tissue engineering and microbial systems. Bioprinting. Volume 18. 2020. https://doi.org/10.1016/j.bprint.2019.e00071.
- Ibrahim T. Ozbolat, Weijie Peng, Veli Ozbolat. Application areas of 3D bioprinting. Drug Discovery Today. Volume 21, Issue 8. 2016. Pages 1257-1271. https://doi.org/10.1016/j.drudis.2016.04.006.
